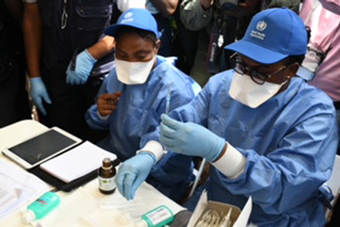
Health workers prepare the first shots of the Ebola vaccine in Mbandaka, DRC. Credit: Gavi/2018/P Barollier.
Geneva, 25 July 2018 – The first use of a vaccine to help contain an Ebola outbreak has been encouraging, Dr Seth Berkley, CEO of Gavi, the Vaccine Alliance, said as the outbreak in the Democratic Republic of Congo (DRC) was officially declared over.
The DRC government formally declared the Ebola outbreak over on Tuesday 24 July after no new cases were reported for 42 days. From the 4 April through 9 July there were 54 confirmed cases of Ebola reported, with 33 deaths. A total of 3,300 people received investigational doses of the vaccine as part of a ring vaccination protocol – the same used to eradicate smallpox.
The vaccination was implemented by the Government of DRC and partners including WHO, which supported national authorities in coordinating the international health response, and Medecins Sans Frontieres (MSF). Gavi provided $1 million towards the vaccination effort.
“As soon as Ebola moved from isolated rural areas into Mbandaka – a major town and regional hub – there was justified concern that this outbreak could spiral out of control,” said Dr Berkley. “It took months of hard work by a global coalition of UN agencies, NGOs and governments, led by the DRC government with WHO support, to carry out the surveillance, containment, contact tracing and public education needed to contain and defeat Ebola. This was the first time a vaccine was used as part of this wider response and it is encouraging that there were no cases of Ebola among those given the vaccine. We also now have valuable experience of how this vaccine can be used effectively in the field.”
The vaccine has gone through Phase 3 trials, which showed the vaccine to be safe and highly effective, but has not yet been licensed by relevant regulatory authorities. While the vaccine goes through the licensing process, an agreement between Gavi and Merck, the developer of the Ebola vaccine, ensures that 300,000 investigational doses of the vaccine are available in case of an outbreak. It is these doses that were used in the DRC.
This agreement, an Advance Purchase Commitment announced in January 2016, is the first of its kind. It was designed to incentivise the rapid development of the vaccine as well as guarantee investigational doses are available while licensure is being secured. Gavi committed US$5 million to buy doses of a fully licensed vaccine as and when it becomes available. In return, Merck agreed to create the emergency stockpile.
“I’m proud of the work Gavi has done to make investigational doses of this vaccine available,” continued Dr Berkley. “Although the emergency stockpile remains available in case of any future outbreaks, we now need to work hard to ensure this vaccine is licensed as quickly as possible.”
Background on the vaccine and the Advance Purchase Commitment
- Between its first documented appearance in 1976 and the 2014–16 epidemic, the Ebola virus infected about 2,400 people and killed fewer than 1,600.
- During this time, scientists researched how to protect against Ebola – a drive mainly fuelled by fears that Ebola could be turned into a bioweapon. Following the terrorist attacks of September 11, 2001 the US dramatically increased biodefence funding. Significant funds were spent developing anti-Ebola vaccines and drugs that could be deployed if the virus was weaponised and intentionally released.
- By 2014, 10 Ebola vaccines and treatments were in various stages of research, development and clinical testing. The USA was not alone in funding biodefence research related to Ebola. Canada’s Department of National Defence invested US$ 7 million in developing an Ebola vaccine at its National Microbiology Laboratory. But no commercial market for Ebola vaccines existed. There was no imperative to create a safe, licensed Ebola vaccine that could be stockpiled and deployed to prevent a large-scale natural outbreak of the disease.
- In August 2014, the same month that the WHO declared an Ebola public health emergency in West Africa, Canada’s federal government donated the vaccine it had researched for biodefence purposes for use in Africa, with the Public Health Agency of Canada licensing its manufacture to NewLink Genetics and Merck.
- In the final days of 2014, the Gavi Board sent a clear signal to manufacturers by committing up to US$ 300 million for the procurement through UNICEF of first generation, licenced Ebola vaccines for the 2014-2016 outbreak and a global stockpile of first generation vaccines for 2016-2020. This support helped encourage health bodies and manufacturers to invest in the accelerated development of candidate vaccines and begin advanced trials.
- In Liberia, the US National Institutes of Health and Liberia Ministry of Health began a randomised, double-blind trial of two experimental vaccines. The first was developed by scientists at GlaxoSmithKline (GSK). The second was the vaccine licensed by Canada to Merck, known as rVSV-ZEBOV. Both of these vaccines elicited an immune response that may protect against the Ebola virus. However, the epidemic ended before the clinical trials could be completed.
- In 2015, Merck’s experimental vaccine was separately tested in Guinea, where health officials implemented the “Ebola, ça suffit” (“Ebola, that’s enough”) vaccination trial. Almost 12,000 people who had come into contact with someone who had showed symptoms of the disease were vaccinated either immediately or after 21 days.
- Guinea was declared Ebola-free on 29 December, 2015. The trial ended on 20 January the following year, after the final participants had completed their 84-day follow-up. The vaccine proved to be well tolerated and effective in adults.
- In 2015, Gavi made a unique offer to all manufacturers that had a vaccine in Phase I clinical trials and beyond, offering a pre-paid commitment to buy doses of licensed vaccines as and when the vaccine becomes available, confirming to the manufacturers that there was a guaranteed market for an effective Ebola vaccine.
- In return Gavi set three conditions: that the manufacturer submits an application for licensure by a set date; that they submit a special classification from the WHO that would allow it to be used in case of a public health emergency; and, most importantly, that they make a stockpile of investigational doses available in case of an outbreak while the vaccine is going through the licensing process.
- In January 2016, Gavi announced at Davos that Merck had agreed to our terms. An advance Purchase Commitment of $5 million – the first of its kind – was signed. This agreement meant that 300,000 investigational doses of the rVSV-ZEBOV vaccine would be available in case of an outbreak, including 100,000 doses that can be shipped within5 calendar days. It is these doses that were used in the DRC.
Media contacts

James Fulker
Gavi
+41 79 429 5505
+41 22 909 2926

Frédérique Tissandier
Gavi
+41 79 300 8253
+41 22 909 2968
