The COVID-19 vaccine race
Scientists around the world are working faster than ever to develop and produce vaccines that can stop the spread of COVID-19, with 21 vaccines now being rolled out in countries worldwide. Here is an at-a-glance overview of those vaccines and recent developments of candidates in clinical trials.
- 12 January 2022
- 74 min read
- by Gavi Staff

The COVID-19 vaccine race – weekly update
Scientists around the world are working faster than ever to develop and produce vaccines that can stop the spread of COVID-19, with 21 vaccines now being rolled out in countries worldwide. Here is an at-a-glance overview of those vaccines and recent developments of candidates in clinical trials.
12 January 2022
Recent updates
-
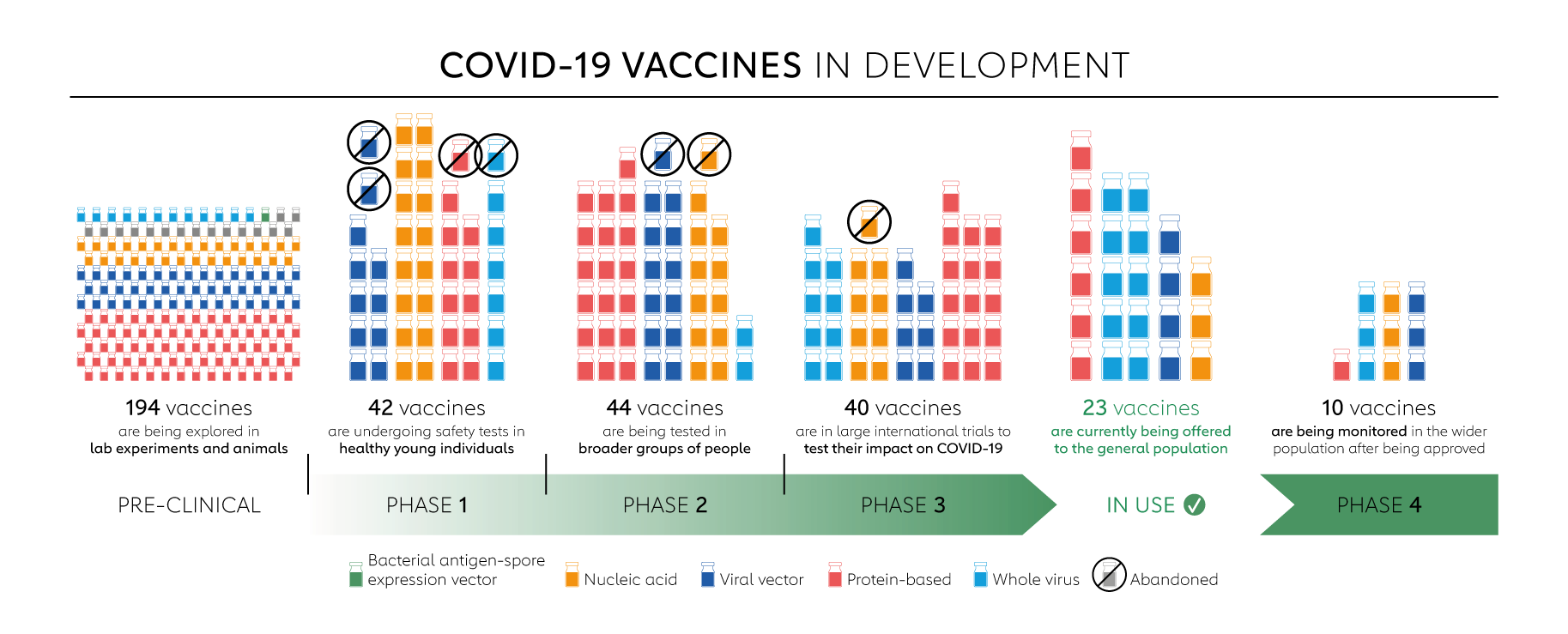
Around the world, there are now 137 COVID-19 vaccine candidates undergoing clinical trials and 194 candidates in pre-clinical development.
-
An oral vaccine developed by DreamTec Research Limited, a Hong Kong based biotechnology company, has completed a study which found that the technology team has succeeded engineering Bacillus subtilis with spore coat proteins resembling the proteins of the nucleus and spikes of coronal virus. This product could have a vaccine like activity within the intestinal environment. This is one of the first examples of a bacterial vector vaccine.
When candidate vaccines make it to human clinical trials, they first go through phase 1 trials primarily to test the vaccine’s safety, determine dosages and identify any potential side effects in a small number of people. Phase 2 trials further explore safety and start to investigate efficacy on larger groups. Phase 3 trials, which few vaccines ever make it to, are much larger, involving thousands or tens of thousands of people, to confirm and assess the effectiveness of the vaccine and test whether there are any rare side effects that only show up in large groups. The final stage, phase 4 trials, is conducted after national regulatory approval and involves further monitoring in a wide population over a longer timeframe as a form of post-marketing surveillance (pharmacovigilance). However, not all vaccines that have been approved for domestic are in phase 4 trials. Regulators in many countries have their own individual procedures and timelines for providing emergency use authorisations, relying on various types of evidence at different clinical trial phases. Some national regulators, including those in Russia and China, began approving vaccines for (limited or widespread) public use even before phase 3 trials were completed. The World Health Organization (WHO) lists candidates at various stages of clinical trials.
Here is a more in-depth look at the candidate vaccines that are in phase 1 trials or beyond.
Have you read?
Filter the different clinical phases
- 4
-
- MEDIGEN (TAIWAN)
PROTEIN SUBUNIT VACCINE
Taiwan-based vaccine maker Medigen have produced a two-dose vaccine made of a combination of spike proteins and an adjuvant from Dynavax. The company began phase 2 trials in adults in late January 2021. In July, a phase 2 trial started on people aged 12 to 18 years old. In the same month, a phase 3 trial was authorised to be carried out in Paraguay. On 19 July, this vaccine was authorised for emergency use in Taiwan. A phase 2 trial was registered on 23 September 2021 to assess the immunogenicity of heterologous prime-boost immunization with AstraZeneca/University of Oxford (AZD1222) vaccine and Medigen (MVC-COV1901) vaccine in adults. The trial has plans to recruit 110 participants and is due to be completed in August 2022. The company started a phase 4 trial on 7 October to measure the level of antibodies produced in adults in order to demonstrate the immunogenicity and safety of the company's vaccine as a booster dose. A phase 4 clinical trial started on 7 October 2021 to measure the anti-SARS-CoV-2 neutralising antibody titers in adult participants in order to demonstrate the immunogenicity and safety of a third booster dose with Medigen's COVID-19 vaccine.
- MODERNA (USA)
RNA VACCINE
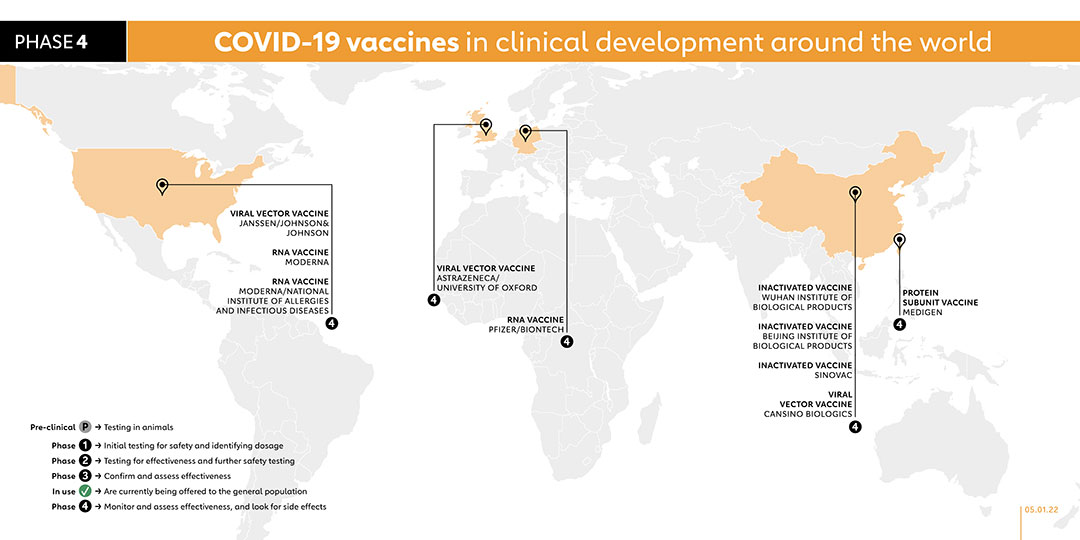
The Moderna vaccine is also known as Spikevax or mRNA-1273. It was funded by the National Institute of Allergy and Infectious Diseases (NIAID), which is part of the US National Institutes of Health. The vaccine is administered in two doses with four weeks apart. It was tested in phase 1 trials on volunteers at the Kaiser Permanente Washington Health Research Institute in Seattle. Moderna has run phase 2 trials on participants of a wide range of ages and started phase 3 trials in July 2020. The final trial enrolled 30,000 healthy people from across the United States. In February, a phase 4 trial was launched as part of a national cohort study in collaboration with the Danish Ministry of the Interior and Health. The United States currently authorises its use for people 18 and older, while the Europe Medicines Agency authorised giving it to children aged 12 to 17 years in July. A phase 2 study was carried out in August 2021 to elicit an antibody response in kidney transplant recipients who have failed to respond to two doses of vaccine. This vaccine needs to be kept in refrigerators, or freezers for longer storage. The Faculty of Medicine Ramagthibodi Hospital in Bangkok, Thailand, under the sponsorship of the National Research Council of Thailand, registered a phase 3 trial in November 2021 to study the immunogenicity of an additional dose of either viral-vectored or mRNA vaccine in kidney transplant recipients. Another phase 3 trial was registered in November 2021 to study the safety and immunogenicity of 9-valent HPV vaccine co-administered with Moderna's SARS-CoV-2 vaccine. A phase 1 trial was registered in November 2021 with the aim to determine the safety, reactogenicity, and immunogenicity of heterologous 3rd booster of mRNA and protein COVID-19 vaccines, including Pfizer/BioNTech's BNT162b2, Moderna's mRNA-1273 and Medigen's MCV COVID-19 vaccine. A phase 3 trial started in October 2021 to to compare short and long-term immunogenicity of different COVID-19 vaccine combinations against the ancestral SARS-CoV-2 as well as different variants of concern. Involving 600 participants, the trial is due to be completed by April 2023. In November 2021, the company registered a phase 2 study to evaluate the immunogenicity and safety of the company's mRNA-1283 COVID-19 vaccine boosters to boost antibody responses to the ancestral strain of SARS-CoV-2 virus, the B.1.351 variant, and potentially other SARS-CoV-2 variants.
- ASTRAZENECA/UNIVERSITY OF OXFORD (UK)
The AstraZeneca vaccine is administered in two doses. It was developed by the University of Oxford, and has been granted emergency use authorisation by the European Medicines Agency, the WHO as well as national regulators worldwide. This vaccine is fridge-stable meaning that it can be easily transported anywhere in the world. At about US$ 4 per dose for lower-income nations, it is also being made available for a fraction of the cost of others . It was tested in phase 3 clinical trials with more than 10,000 people from across the UK, including children and older people. The vaccine was also tested in Brazil, the United States and India and South Africa started the first COVID-19 vaccine trial in Africa. In February 2021, a phase 4 trial was launched as part of a national cohort study in collaboration with the Danish Ministry of the Interior and Health. A phase 2/3 trial was started on 1 June 2021 to study the effectiveness, safety and immunogenicity of a half dose of the vaccine. On 27 September 2021, the company registered a phase 4 trial to assess the immunogenicity and safety of the vaccine for prevention of COVID-19 in immunocompromised adults. A phase 2 trial was registered on 21 October 2021 to compare the immunogenicity and safety of heterologous and homologous booster schedules in individuals who received AstraZeneca's ChAdOx1-S or Sinovac's CoronaVac vaccination previously. With plans to enrol 520 participants, the study is due to be conducted from 1 December 2022. A phase 1/2 study was registered in November 2021 to evaluate and compare the safety and immunological response of the third dose of AstraZeneca’s ChAdOx1 vaccine and Pfizer’s mRNA vaccine. The Faculty of Medicine Ramagthibodi Hospital in Bangkok, Thailand, under the sponsorship of the National Research Council of Thailand, registered a phase 3 trial in November 2021 to study the immunogenicity of an additional dose of either viral-vectored or mRNA vaccine in kidney transplant recipients.
A phase 1/2 study was registered in November 2021 to evaluate and compare the safety and immunological response of the third dose of AstraZeneca’s ChAdOx1 vaccine and Pfizer’s mRNA vaccine. The Faculty of Medicine Ramagthibodi Hospital in Bangkok, Thailand, under the sponsorship of the National Research Council of Thailand, registered a phase 3 trial in November 2021 to study the immunogenicity of an additional dose of either viral-vectored or mRNA vaccine in kidney transplant recipients. A phase 1/2 trial was registered on 24 November 2021. The longitudinal study will include health professionals and patients with immune-mediated inflammatory diseases (IMID) who will receive the AstraZeneca ChAdOx1 nCoV-19 vaccine, to assess the safety, efficacy and duration of the short- and long-term humoral and cellular immune response after vaccination and compare the vaccine response between individuals who have or have not had previous COVID-19 infection. A phase 1 trial was registered in November 2021 with the aim to determine the safety, reactogenicity, and immunogenicity of heterologous 3rd booster of mRNA and protein COVID-19 vaccines, including Pfizer/BioNTech's BNT162b2, Moderna's mRNA-1273 and Medigen's MCV COVID-19 vaccine.
In March 2021, the University of Oxford registered a further phase 1 trial in the UK with 30 adult participants to investigate the delivery of its ChAdOx1 vaccine using a nasal spray. ChAdOx1 is currently being delivered by intramuscular injection as part of the UK’s national rollout. By using a different technique that administers the vaccine to the site of infection, researchers at Oxford intend to investigate whether this results in enhanced protection, especially against transmission and mild disease. On 27 June, a new phase 2/3 trial was carried out to demonstrate the safety and characterise the immunogenicity of the Beta variant vaccine, called AZD2816. A phase 2/3 study result was published on 29 November 2021. Aimed to assess the safety and immunogenicity of the AstraZeneca’s SII-ChAdOx1 vaccine in adults in India, the study found that the vaccine has a non-inferior immune response compared to the company's AZD1222 vaccine and an acceptable safety/reactogenicity profile. A phase 2 trial was registered in December 2021 aims to evaluate the immunogenicity, safety and reactogenicity of different SARS-CoV-2 vaccines namely Sinopharm's BIBP, CanSinoBIO and AstraZeneca ChAdOx.
- PFIZER/BIONTECH (GERMANY)
RNA VACCINE
The vaccine is administered in two doses that are given three weeks apart. In December 2020, the UK became the first country in the world to approve this vaccine and began rolling out an initial 800,000 doses at the start of the month, and is now in use worldwide. BioNTech, working together with Pfizer, started testing its BNT162 vaccine in humans in global trials, initially in Germany, and then started trials in the USA. BioNTech has also entered into a € 100 million debt financing agreement with the European Investment Bank in order to scale-up the production of the vaccine in Europe. On 27 July 2020, it announced the launch of a phase 2/3 trial with 30,000 volunteers in the USA and other countries including Argentina, Brazil and Germany. In September, it said it would expand its phase 3 US trial to 44,000 participants. At the start of October 2020, BioNTech and Pfizer started recruiting for a phase 3 trial in South Africa and by early November had reported promising interim results. Its final efficacy analysis confirmed strong efficacy. In February, a phase 4 trial was launched as part of a national cohort study in collaboration with the Danish Ministry of the Interior and Health. The United States Food and Drug Administration (FDA) has authorised its use for 12 to 15 years olds in the United States. On 23 August 2021, the FDA granted full approval to the vaccine for people 16 and older, moving it beyond the emergency-use status in the US – the first vaccine to do so. A phase 2 study was carried out in August 2021 to elicit an antibody response in kidney transplant recipients who have failed to respond to two doses of vaccine. On 12 August 2021, the United States FDA authorised the use of booster shot in transplant recipients. This vaccine requires freezer storage. On 27 September 2021, a phase 4 trial was registered to study a third dose of the vaccine to boost COVID-19 immunity. On the same day, a separate phase 4 trial was registered to study the comparison effects of third dose with Sinovac or mRNA vaccine (Comirnaty) to boost the immune response in adults who previously received two doses of Sinovac or BNT162b2 (Comirnaty, BioNTech/Fosun Pharma). A phase 4 trial was registered on 12 October 2021 and expects to enrol 525 participants. The study is expected to start in November 2021. This trial aims to measure the humoral and adaptive immune response in patients with cancer diagnosis undergoing mRNA vaccination against SARS-CoV-2. On 14 October 2021, a phase 2 trial was registered with plans to enrol 400 participants. The purpose of the study is to induce an enhanced antibody response to COVID-19 in kidney and liver transplant recipients who have negative or indeterminate anti-spike antibody after at least two Moderna or Pfizer-BioNTech mRNA COVID-19 vaccines. Participants will be randomized to receive a Moderna or Pfizer-BioNTech COVID-19 booster dose, or receive the booster dose together with a temporary, prescribed reduction in their maintenance immunosuppression (IS) regimen. In November 2021, a study was registered to evaluate the effect of sirolimus-based immunosuppression and inulin dietary fibre supplementation on booster COVID-19 vaccine responses in kidney transplant recipients. A phase 1/2 trial was registered in November 2021 to evaluate and compare the safety and immunological response of the third dose of AstraZeneca’s ChAdOx1 vaccine and Pfizer’s mRNA vaccine. On 17 November 2021, a phase 2 trial has started to evaluate the immunogenicity after a Pfizer booster dose among elderly or adult with underlying diseases who had received AstraZeneca’s AZD1222 vaccine using standard versus low dose. The Faculty of Medicine Ramagthibodi Hospital in Bangkok, Thailand, under the sponsor of the National Research Council of Thailand, registered a phase 3 trial in November 2021 to study the immunogenicity of an additional dose of either viral-vectored or mRNA vaccine in kidney transplant recipients. A phase 3 trial was registered in November 2021. The objective of this trial is to evaluate the immunogenicity and safety induced by a homologous vaccine booster (Pfizer-BioNTech vaccine booster) and a heterologous vaccine booster (one of the two experimental Sanofi/GSK vaccines booster), on the D614 (Wuhan) strain and on the SARS-CoV-2 variants, in adults who received 2 doses of Pfizer-BioNTech mRNA vaccine in November 2021 to study the immunogenicity of an additional dose of either viral-vectored or mRNA vaccine in kidney transplant recipients. A phase 1 trial was registered in November 2021 with the aim to determine the safety, reactogenicity, and immunogenicity of a 3rd booster of mRNA and protein COVID-19 vaccines, including Pfizer/BioNTech's BNT162b2, Moderna's mRNA-1273 and Medigen's MCV COVID-19 vaccine. A trial was registered in November 2021 to evaluate the post-vaccination immune response to COVID-19 in the New Caledonian Population (COVCAL). The importance of the study lies in the fact that the response of non-European non-Asian Oceanian populations to Pfizer vaccination has not been specifically studied at this time. Some recent data are in favour of a significant variability of susceptibility to pathogens in Oceanian populations, stemming from a genetic inheritance from Neanderthal man and his cousin Denisova man. In the context of vaccine hesitancy, it is therefore important to ensure that the immune response of the New Caledonian population (Melanesian, Polynesian, European or other communities) to vaccination against COVID-19 is similar to that of populations studied in large clinical trials.
A phase 3 trial was started in October 2021 to compare short and long-term immunogenicity of different COVID-19 vaccine combinations against the ancestral SARS-CoV-2 as well as different variants of concern. Involving 600 participants, the trial is due to be completed by April 2023. A phase 1/2 trial was started in November 2021 to study the immune response of heterologous boost third dose of mRNA and protein COVID-19 vaccine, including Pfizer's BNT162b2, Moderna's mRNA-1273 and Medigen's COVID-19 vaccine.
- SINOVAC (CHINA)
INACTIVATED VACCINE
Sinovac conducted phase 3 trials involving volunteers in Brazil, Indonesia and Turkey. Although it is not yet approved by regulators, shipments have already arrived in Indonesia, ready for rollout. A report in July said that the Chinese government has given the Sinovac vaccine emergency approval for limited use. The city of Jiaxing has reportedly offered the vaccine to health workers and other high-risk groups for US$ 60. The company began phase 4 trials in February 2021. A Phase 2 study was carried out on 12 July 2021 to determine the safety and immunogenicity of booster doses. A phase 3 study is due to be conducted in late August 2021 to evaluate the efficacy of the vaccine in participants aged 6 months to 17 years. A study report on a phase 4 clinical trial was published on 23 August 2021 which found that simultaneous administration of the vaccine and seasonal influenza vaccine would be feasible. On 27 September 2021, a phase 4 trial was registered to study the comparison effects of a 3rd dose with Sinovac or mRNA vaccine (Comirnaty) to boost the immune response in adults who previously received two doses of Sinovac or BNT162b2 (Comirnaty, BioNTech/Fosun Pharma). A phase 3 trial was registered on 14 October 2021 to evaluate the efficacy, safety, and immunogenicity of two inactivated vaccines as booster dose, CoronaVac and Turkovac, in individuals who received the second dose of Sinovac's CoronaVac vaccine after 90 days to 270 days. On 21 October 2021, a phase 2 trial was registered to compare the immunogenicity and safety of heterologous and homologous booster schedules in individuals who received AstraZeneca's ChAdOx1-S or Sinovac's CoronaVac vaccination previously. With plans to enrol 520 participants, the study is due to be conducted on 1 December 2022. A phase 3 trial was started in October 2021 to compare short and long-term immunogenicity of different COVID-19 vaccine combinations against the ancestral SARS-CoV-2 as well as different variants of concern. Involving 600 participants, the trial is due to be completed by April 2023. In November 2021, the company registered a phase 4 clinical trial in children aged 3-5 years old. The purpose of this study is to evaluate the immunogenicity and safety of the company's COVID-19 vaccine (Vero cell) when co-administered with the EV71 vaccine manufactured by Sinovac Biotech Co. Another phase 3 trial was registered in November 2021 to evaluate the company's COVID-19 vaccine’s use in healthy populations aged from 3 to 11 years against that in adults aged 18-26 years. The company registered a phase 2 trial in December 2021 to evaluate the immunogenicity of using the high or medium dose of CoronaVac vaccine as the third booster dose in adults in Turkey. The company also registered two phase 4 trials in December 2021. One of the phase 4 trials aims to explore booster immunisation of SARS-CoV-2 inactivated vaccine from different manufactures in adults previously vaccinated with inactivated COVID-19 vaccine. Vaccines manufactured by Sinovac Research & Development Co Ltd, Beijing institute of Biological Products Co Ltd and Wuhan Institute of Biological Products Co Ltd will be studied. Another phase 4 trial sponsored by Jiangsu Province Centers for Disease Control and Prevention aims to evaluate the safety and immunogenicity of three doses of CoronaVac's inactivated COVID-19 vaccine in Chinese pulmonary tuberculosis adult patients.
- CANSINO BIOLOGICS INC. (CHINA)
The Ad5-nCoV vaccine candidate uses a harmless non-replicating viral vector to carry vaccine antigens into the human body – this is the same platform that the vaccine developer CanSino Biologics Inc, based in Tianjin, used for its Ebola vaccine. The COVID-19 vaccine was jointly developed with the Institute of Biotechnology of the Academy of Military Medical Sciences. On 25 June 2020, the Chinese military approved the vaccine for a year as a “specially needed drug”, which is unusual given that at that point phase 2 results had not been collated. On 9 August 2020, the Saudi health ministry announced that CanSino would run a phase 3 trial in Saudi Arabia; later in the month the company also started a trial in Pakistan and Russia. In May 2021, the company registered a phase 4 trial with 300 adult participants who have been primed with either one or two doses of inactive SARS-CoV-2 vaccine. On 5 August 2021, Reuters reported that antibody levels in people inoculated with this vaccine could drop to around 30 percent after six months. A phase 2 trial report published on 22 September 2021 confirmed a single dose of the vaccine was safe and induced robust immune responses in individuals aged 6-17 years. A total of 2000 participants will be equally divided into the two study countries, Chile and Mexico. All participants will receive a 1st dose of intramuscular Ad5-nCoV and a 2nd dose of inhaled Ad5-nCoV-IH. A phase 2 trial was registered in December 2021 aims to evaluate the immunogenicity, safety and reactogenicity of different SARS-CoV-2 vaccines namely Sinopharm's BIBP, CanSinoBIO and AstraZeneca ChAdOx.
- BEIJING INSTITUTE OF BIOLOGICAL PRODUCTS (CHINA)
INACTIVATED VACCINE
The Beijing Institute is part of China’s state-run Sinopharm Group, and developed its vaccine called BBIBP-CorV, in collaboration with the Chinese Center for Disease Control and Prevention. In phase 3 trials in the UAE, 5,000 people received BBIBP-CorV. The Institute has launched a phase 4 trial for the vaccine. A phase 3 trial is due to be conducted in October 2021 to assess the safety, immunogenicity and efficacy of two doses of the vaccine, followed by a booster dose in adults 18 years of age and older. A phase 4 trial was started on 8 October 2021 involving 400 participants. The purpose of this trial is to study the immunogenicity and safety of the company's vaccine in elderly people with chronic bronchitis and chronic obstructive pulmonary disease. Two phase 4 trials were registered in November 2021. The purpose of the trials is to evaluate the post-marketing immunogenicity, safety and antibody persistence of the third dose (booster) of the company’s COVID-19 vaccine, produced in Wuhan and Beijing, in patients aged 60 years or older with hypertension and/or diabetes. Another phase 4 trial was registered in November 2021 to evaluate the immunogenicity, safety and persistence of the third dose of inactivated COVID-19 vaccine in individuals infected with HIV. A separate phase 4 trial was also registered in November to evaluate the immunogenicity, safety and persistence of a third dose of the vaccine in individuals aged 60 years and above with chronic bronchitis and chronic obstructive pulmonary disease. A trial was registered on 29 November 2021 to compare the safety and immunogenicity of FAKHRAVAC and Sinopharm booster doses for adults who are fully vaccinated by Sinopharm. A phase 2 trial was registered in December 2021 to evaluate the safety and immunogenicity of SARS-COV-2 vaccine (Vero Cell) in adults who have received the 2 prime doses of one of SARS-COV-2 Vaccines (Vero Cell inactivated-Sinopharm SARS-COV-2 Vaccine, CoronaVac SARSCOV-2 Vaccine, AstraZeneca SARS-COV-2 Vaccine, or Cominarty/Pfizer mRNA COVID-19 Vaccine). Another phase 2 trial registered in December 2021 aims to evaluate the immunity, safety and reactogenicity of different SARS-CoV-2 vaccines namely Sinopharm's BIBP, CanSinoBIO and AstraZeneca ChAdOx.
- JANSSEN/JOHNSON&JOHNSON (USA)
J&J has developed vaccines for Ebola and other diseases with Recombinant Adenovirus Serotype 26 (Ad26) and has now made one for the coronavirus. It launched phase 1/2 trials in July 2020, and in September a phase 3 trial with 60,000 participants in Latin America. It hopes to make up to a billion doses in 2021. Janssen began phase 3 trials in the UK in November last year. J&J has committed 500 million doses of this vaccine to the COVAX initiative for distribution worldwide. In January, the company announced that the vaccine had an efficacy of 66% in Latin America, 57% in South Africa and 72% in the United States, with 100% efficacy against severe disease in all trials. In June 2021, the company launched a phase 4 trial in the Netherlands. J&J is among the vaccines in the COVAX portfolio. It is known that many persons, including a significant number of persons with multiple sclerosis (PWMS), who take medications that chronically suppress the immune system do not produce neutralizing antibodies to COVID-19 proteins in response to vaccination. To test this, a phase 4 trial was registered on 18 October 2021 to see whether giving booster doses of COVID-19 vaccine to persons with multiple sclerosis can improve the immune response to COVID-19. A phase 3 trial was registered on 25 October 2021. A recent study report published on 22 October 2021 proved that the J&J vaccine was effective as a booster. A phase 1/2 trial registered in November 2021 aims to address evidence gaps regarding the safety, reactogenicity and immune responses of a heterologous boost of a single dose of J&J vaccine (half or full dose) in recipients who have received either 1-dose or 2-doses of Sinovac and/or Sinopharm’s inactivated COVID-19 vaccines. A phase 3 trial was started in October 2021 to compare short and long-term immunogenicity of different COVID-19 vaccine combinations against the ancestral SARS-CoV-2 as well as different variants of concern. Involving 600 participants, the trial is due to be completed by April 2023.
- MODERNA/NATIONAL INSTITUTE OF ALLERGIES AND INFECTIOUS DISEASES (US)
RNA VACCINE
In collaboration with the National Institute of Allergies and Infectious Diseases (NIAID), US-based Moderna initially launched a phase 1 trial to test their second COVID-19 vaccine, mRNA-1273.351. The vaccine specifically targets the SARS-CoV-2 Beta variant, which was first identified in the Republic of South Africa. The mRNA-1273.351 vaccine candidate will be given to trial participants in vaccination schedules alone, sequentially, or co-administered with Moderna’s first candidate, mRNA-1273, which has already been approved and rolled out in the US. A phase 2 trial, which is also testing the efficacy of the vaccine against COVID-19 in cancer patients, was launched in April 2021 with 120 participants. In June, Moderna launched a phase 4 trial for mRNA-1273.351 in Belgium. On 11 August 2021, results from a phase 2/3 trial suggested that the vaccine was safe to use and efficacious in preventing COVID-19 in adolescents between 12 and 17 years old. On the same day, results from a phase 3 trial of a third-dose vaccine in adult transplant recipients showed that the booster enhanced their immune response. The United States Food and Drug Administration has authorised the use of booster shot in transplant recipients. A phase 4 trial was registered on 1 September 2021 to study the efficacy of booster vaccination in kidney transplant patients who did not sero-convert after two doses of vaccine. A phase 4 trial was registered on 12 October 2021 and expects to enrol 525 participants. The study is expected to start in November 2021. This trial aims to measure the humoral and adaptive immune response in patients with cancer diagnosis undergoing mRNA vaccination against SARS-CoV-2. On 14 October 2021, a phase 2 trial was registered with plans to enrol 400 participants. The purpose of the study is to induce an enhanced antibody response to COVID-19 in kidney and liver transplant recipients who have negative or indeterminate anti-spike antibody after at least two Moderna or Pfizer-BioNTech mRNA COVID-19 vaccines. Participants will be randomized to receive a Moderna or Pfizer-BioNTech COVID-19 booster dose, or receive the booster dose together with a temporary, prescribed reduction in their maintenance immunosuppression (IS) regimen. In November 2021, the company registered a phase 2 study to evaluate the immunogenicity and safety of the company's mRNA-1283 COVID-19 vaccine boosters to boost antibody responses to the ancestral strain of SARS-CoV-2 virus, the B.1.351 variant, and potentially other SARS-CoV-2 variants.
- WUHAN INSTITUTE OF BIOLOGICAL PRODUCTS (CHINA)
INACTIVATED VACCINE
The United Arab Emirates became the first foreign country to approve this vaccine, after interim phase 3 trials showed 86% efficacy. The Wuhan Institute launched phase 3 trials in July 2020 in the UAE, and in Morocco and Peru in August 2020. The state-owned Chinese company Sinopharm has been putting the vaccine through clinical tests. On 14 Sept, the UAE gave emergency approval for the vaccine to be given to health care workers. Wuhan Institute of Biological Products registered the vaccine for a phase 4 trial with 1,440 participants in October 2021.
- In use
-
- NOVAVAX (USA)
PROTEIN SUBUNIT VACCINE
Novavax is using a nanoparticle technology platform to generate antigens from the spike protein found on the outer shell of the coronavirus. The vaccine, NVX-CoV2373, also received the highest funding from CEPI, with a total of US$ 388 million. Phase 2 trials started in August 2020 in South Africa and phase 3 trials started in the UK in September as well as in the USA in December 2020.
In January, the company announced that its vaccine had an efficacy rate of 89.3% based on trials in the United Kingdom. In South Africa, Novavax’s vaccine efficacy rate was lower at below 50%, potentially due to the dominance of the Beta COVID-19 variant in South Africa, which can evade the antibodies stimulated by the vaccine. In both cases the vaccine was found to be 100% effective against severe disease. NVX-CoV2373 is among the vaccines within the COVAX portfolio. In November 2021, the company registered a phase 2 trial to evaluate the safety and immunogenicity of the company's vaccine in people living with HIV. A phase 2 trial study report was published in December 2021. The trial studied the safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of AstraZeneca's ChAdOx1 vaccine or Pfizer's BNT162b2 in the UK.
- ORGANIZATION OF DEFENSIVE INNOVATION AND RESEARCH (IRAN)
INACTIVATED VACCINE
In March, the Organization of Defensive Innovation and Research, a subsidiary of Iran’s Ministry of Defense, launched a phase 1 trial for the country’s third locally developed vaccine, FAKHRAVAC (MIVAC). FAKHRAVAC, which was tested at two different dose strengths each injected as part of a two dose schedule two and three weeks apart, is named after Mohsen Fakhrizadeh, one of Iran’s top nuclear scientists who was killed in late November 2020. In June 2021, FAKHRAVAC underwent a phase 2 trial. Although FAKHRAVAC gained emergency authorisation in September, Iran announced that it would be abandoning the vaccine in October 2021 due to lack of demand.
- ASTRAZENECA/UNIVERSITY OF OXFORD (UK)
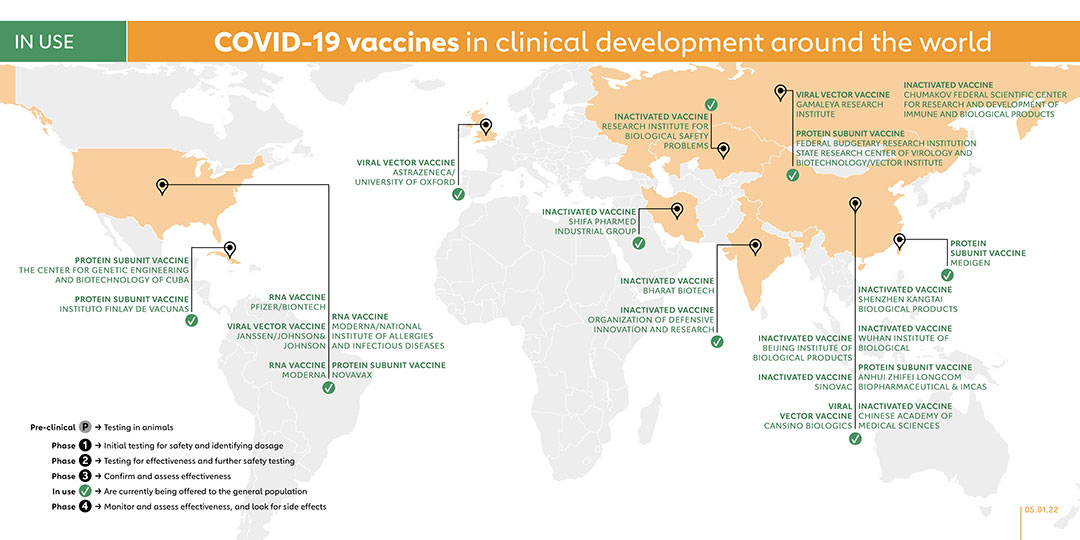
The ChAdOx1 vaccine, developed by AstraZeneca/University of Oxford, has a vaccine efficacy of up to 90%. The United Kingdom and Argentina were the first countries to give the vaccine emergency authorisation in December 2020. They were followed by India (which approved a version of ChAdOx1 called Covishield made by the Serum Institute of India), Brazil, Mexico, Pakistan as well as the European Medicines Agency. In February, the World Health Organization (WHO) granted an Emergency Use Listing (EUL) for two versions of the AstraZeneca/Oxford COVID-19 vaccine, produced by AstraZeneca-SKBio (Republic of Korea) and the Serum Institute of India. Covishield is among the vaccines in the COVAX portfolio.
- PFIZER/BIONTECH (GERMANY)
RNA VACCINE
In its final efficacy analysis, BioNTech and Pfizer’s data showed a vaccine efficacy rate of 95%. On 2 December 2020, the United Kingdom became the first country in the world to grant emergency authorisation to Pfizer and BioNTech’s vaccine, and this move has been followed by many more countries. On 31 December 2020, the WHO granted the Pfizer/BioNTech vaccine, BNT162, an Emergency Use Listing, making it the first vaccine to be approved by WHO since the pandemic was declared. BNT162b2 is among the vaccines in the COVAX portfolio.
- MODERNA (USA)
RNA VACCINE
Moderna started phase 3 trials in July 2020 and the final trial results confirmed the company’s vaccine, mRNA-1273, has a 94% efficacy rate. On 18 December 2020, it became the second COVID-19 vaccine authorised by the Food and Drug Administration (FDA) in the United States, coming a week after the vaccine made by Pfizer and BioNTech was authorised. On 30 April 2021, mRNA-1273 became the fifth vaccine to receive emergency validation from WHO. MRNA-1273 is among the vaccines in the COVAX portfolio.
- JANSSEN/JOHNSON&JOHNSON (USA)
In January, the company announced that its vaccine, Ad26.COV2.S, had an efficacy of 66% in Latin America, 57% in South Africa and 72% in the United States, with 100% efficacy against severe disease in all trials. Bahrain became the first country to authorise the vaccine for emergency use on 25 February 2021, with the FDA following suit on 27 February and making the Johnson & Johnson’s vaccine third COVID-19 vaccine (and first single-dose vaccine) available in the United States. On 12 March 2021, WHO listed the Johnson & Johnson vaccine for emergency use in all countries and for COVAX roll-out, a day after the vaccine was given authorisation by the European Medicines Agency (EMA). A phase 4 trial was registered on 1 September 2021 to study the efficacy of booster vaccination in kidney transplant patients who did not sero-convert after two doses of vaccine. On 20 September 2021, a phase 4 trial was carried out to evaluate the immune response in two age groups: over 65s and 55 to 65 years. With plans to recruit 120 participants, this study is due to be completed in October 2023.
- GAMALEYA (RUSSIA)
On 2 February 2021, the company released the results of its phase 3 trial, including an efficacy rate of 91.6% for its non-replicating viral vector vaccine candidate known as Sputnik V. Back in November 2020, the Russian government had already began offering Sputnik V to the general population. On 22 December that year, Belarus became the first country outside of Russia to register Sputnik V. Since then, other countries have followed suit.
- FEDERAL BUDGETARY RESEARCH INSTITUTION STATE RESEARCH CENTER OF VIROLOGY AND BIOTECHNOLOGY/VECTOR INSTITUTE (RUSSIA)
PROTEIN SUBUNIT VACCINE
The Vector Institute developed a COVID-19 vaccine called EpiVacCorona and are currently in phase 3 trials. On 14 October 2020, President Vladimir Putin announced that Russia had granted regulatory approval for EpiVacCorona, making it the second COVID-19 vaccine to be approved in Russia, following Gamaleya’s Sputnik V vaccine. Belarus and Turkmenistan have also authorised EpiVacCorona, which is administered in 2 doses across 21 days.
- CHUMAKOV FEDERAL SCIENTIFIC CENTER FOR RESEARCH AND DEVELOPMENT OF IMMUNE AND BIOLOGICAL PRODUCTS (RUSSIA)
INACTIVATED VIRUS VACCINE
On 20 February 2021, the third domestic vaccine against COVID-19 was approved for domestic use in Russia. The vaccine, "CoviVac," was developed by the Federal Scientific Center for Research and Development of Immune and Biological Products.
- BHARAT BIOTECH (INDIA)
INACTIVATED VACCINE
In collaboration with the Indian Council of Medical Research and the National Institute of Virology, Bharat Biotech designed a vaccine called Covaxin based on an inactivated form of the coronavirus. On 3 January 2021, the Indian government granted emergency use authorisation to Covaxin. As of June 2021, Covaxin received Emergency Use Authorisations (EUA) in 16 countries including the Philippines, Brazil, India, Mexico and Iran, alongside the WHO’s acceptance of Bharat Biotech’s expression of interest (EoI) for Emergency use Listing.
- SINOVAC (CHINA)
INACTIVATED VACCINE
Sinovac conducted phase 3 trials for its inactivated vaccine, known as CoronaVac, with volunteers in Brazil, Indonesia, and Turkey. In January 2021, Indonesia, Turkey, and Brazil authorised the vaccine for use and in February, Sinovac announced that its vaccine had been approved for public use in China. Several Phase 4 trials have been launched since February, 2021. On 1 June 2021, WHO authorised the Sinovac-CoronaVac vaccine for emergency use, while the European Medicines Agency has begun a rolling review of CoronaVac. CoronVac has been authorised by 42 countries and is administered in 2 doses over a 14-day period. CoronaVac is among the vaccines in the COVAX portfolio.
- CANSINO BIOLOGICS INC. (CHINA)
The non-replicating viral vector vaccine, known as Ad5-nCoV, from CanSino Biologics was jointly developed with the Institute of Biotechnology of the Academy of Military Medical Sciences. On 25 June 2020, the Chinese military Chile, China, Ecuador, Hungary, Malaysia, Mexico, Moldova, Pakistan approved the vaccine for a year as a “specially needed drug,” which was unusual given that at that point phase 2 results had not been collated. In February 2021, China announced the approval of the Ad5-nCoV vaccine for general use while Hungary’s National Institute of Pharmacy and Nutrition granted the approval for public use of CanSino’s vaccine in March, making it the second Chinese-made vaccine to receive approval in the country. In June, Argentina similarly granted the vaccine emergency approval.
- BEIJING INSTITUTE OF BIOLOGICAL PRODUCTS (CHINA)
INACTIVATED VACCINE
The Beijing Institute is part of China’s state-run Sinopharm Group, and developed its vaccine called BBIBP-CorV, in collaboration with the Chinese Center for Disease Control and Prevention. China gave Sinopharm emergency approval in the summer of 2020 while the United Arab Emirates (UAE) granted the vaccine approval in December. Since then, several other countries have authorised the vaccine, including Hungary, which was the first European country to offer a Chinese-made vaccine to its population. The WHO granted emergency use authorisation to BBIBP-CorV in May 2021. In May 2021, BBIBP-CorV launched Phase 4 trials of different 3 dose-schedules for those aged 60 years old and above, as well as 3-17 years old and a 2-dose schedule for those aged 18-59 years old. BBIBP-CorV is among the vaccines in the COVAX portfolio.
- WUHAN INSTITUTE OF BIOLOGICAL PRODUCTS (CHINA)
INACTIVATED VACCINE
The Wuhan Institute, part of the state-owned Chinese company Sinopharm, launched phase 3 trials for its vaccine in July 2020 in the UAE, and in Peru and Morocco in August 2020. On 25 February 2021, China announced the approval of the Wuhan vaccine for general use. The UAE subsequently became the first foreign country to approve the vaccine.
- SHENZHEN KANGTAI BIOLOGICAL PRODUCTS/ BEIJING MINHAI BIOTECHNOLOGY CO., LTD. (CHINA)
INACTIVATED VACCINE
The Chinese manufacturing partner of AstraZeneca, Shenzhen Kangtai Biological Products, has its own inactivated COVID-19 vaccine, known as Vero Cells. The phase 2 trials established 18 to 59 years as suitable age groups for the vaccine. In April 2021, the company registered a phase 3 trial, which was launched in May. In that same month, the Chinese government granted the vaccine emergency use approval.
- CHINESE ACADEMY OF MEDICAL SCIENCES, INSTITUTE OF MEDICAL BIOLOGY (CHINA)
INACTIVATED VACCINE
Phase 2 trials began in July 2020 and was followed by a phase 3 trial in 29,000 participants in December 2020. In June 2021, the vaccine was granted emergency use authorisation in China and it is estimated that 500 million to 1 billion doses will be produced annually with a recent expansion of factory production.
- ANHUI ZHIFEI LONGCOM BIOPHARMACEUTICAL, INSTITUTE OF MICROBIOLOGY OF THE CHINESE ACADEMY OF SCIENCES (CHINA/UZBEKHISTAN)
PROTEIN SUBUNIT VACCINE
The Chinese company Anhui Zhifei Longcom and the Institute of MedicalBiology at the Chinese Academy of Medical Sciences partnered to make a vaccine. The vaccine is in Phase 3 trials with 29,000 volunteers. China authorised the vaccine for emergency use in March 2021, making it the fifth COVID-19 vaccine approved in the country and the fourth to be given emergency use approval in the country. It has also been authorised by Uzbekhistan. The vaccine consists of three doses administered in monthly intervals. It can be stored at normal refrigeration temperatures.
- RESEARCH INSTITUTE FOR BIOLOGICAL SAFETY PROBLEMS (KAZAKHSTAN)
INACTIVATED VACCINE
Kazakhstan began research on a vaccine made from inactivated coronaviruses over the Summer. On 1 September 2020 it started phase 2 trials of its vaccine known as QazVac. On 19 December, it reported that phase 2 trials had been completed, with participants producing an immune response. The researchers launched a phase 3 trial and in April 2021, Kazakhstan began administering the vaccine to the general public and donated 25,000 doses to Kyrgyzstan in July, 2021. QazVac is recommended for individuals over 18 years with two doses administered across a 21-day period.
- SHIFA PHARMED INDUSTRIAL GROUP (IRAN)
INACTIVATED VACCINE
Iran-based Shifa Pharmed Industrial Co. created a vaccine called COVIran Barekat and launched a phase 2/3 trial with 20,000 volunteers in six cities including Tehran. A phase 1/2 trial was registered in November 2021 to compare the tolerability, safety, and immunogenicity of Shifa-Pharmed's vaccine (CovIran) and Sinopharm's vaccine in the healthy population aged 12 to 18 years.
- THE CENTER FOR GENETIC ENGINEERING AND BIOTECHNOLOGY OF CUBA (CUBA)
PROTEIN SUBUNIT VACCINE
Along with its nasal spray vaccine, the Center for Genetic Engineering and Biotechnology of Cuba also launched a separate phase 1/2 trial in November 2020 for a vaccine called Abdala, which is injected into the muscle. In February 2021, the Center launched phase 2 trials for the Abdala vaccine, and in March a phase 3 trial, with a planned recruitment of more than 40,000 participants. The vaccine is authorised for emergency use in Cuba and Venezuela.
- INSTITUTO FINLAY DE VACUNAS (CUBA)
PROTEIN SUBUNIT VACCINE
In October 2020, Instituto Finlay De Vacunas launched trials on its second COVID-19 vaccine, Soberana 2. In December 2020, it launched a phase 2 trial and reached an agreement with the Pasteur Institute of Iran in January 2021 to test the vaccine in phase 3 trials. In March, a phase 3 trial was registered for Soberana 2. The vaccine has been used in Cuba and authorised for emergency use in Iran.
- MEDIGEN (TAIWAN)
PROTEIN SUBUNIT VACCINE
Taiwan-based vaccine maker Medigen made a two-dose vaccine made of a combination of spike proteins and an adjuvant from Dynavax. The company began phase 2 trials in late January 2021 and expects to enrol 3,700 participants aged 20 years and over. In July, a phase 2 trial started on people aged 12 to 18 years old. In the same month, a phase 3 trial was authorised in Paraguay. On 19 July, this vaccine was authorised for emergency use in Taiwan. World Health Organisation has included this vaccine in their phase 3 trial of vaccines.
- ZYDUS CADILA (INDIA)
DNA VACCINE
On 3 July Zydus Cadila announced approval to start human phase 1/2 trials. Phase 2 trials launched in August 2020. Zydus Cadila is now initiating a phase 3 trial with 300,000 participants. On 20 August, Zydus Cadila received emergency authorization from the Indian government.
- VAXINE PTY LTD./CINNAGEN CO. (AUSTRALIA/IRAN)
PROTEIN SUBUNIT VACCINE
Vaxine launched a phase 1 trial in July 2020. Its vaccine, called SpikoGen, combines viral proteins with an adjuvant that stimulates immune cells. In May 2021, the company registered a phase 2 trial in Iran with vaccine makers, CinnaGen Co, followed by a phase 3 trial registered in August 2021 to evaluate efficacy and safety of the vaccine. SpikoGen has been authorised by Iran for emergency use.
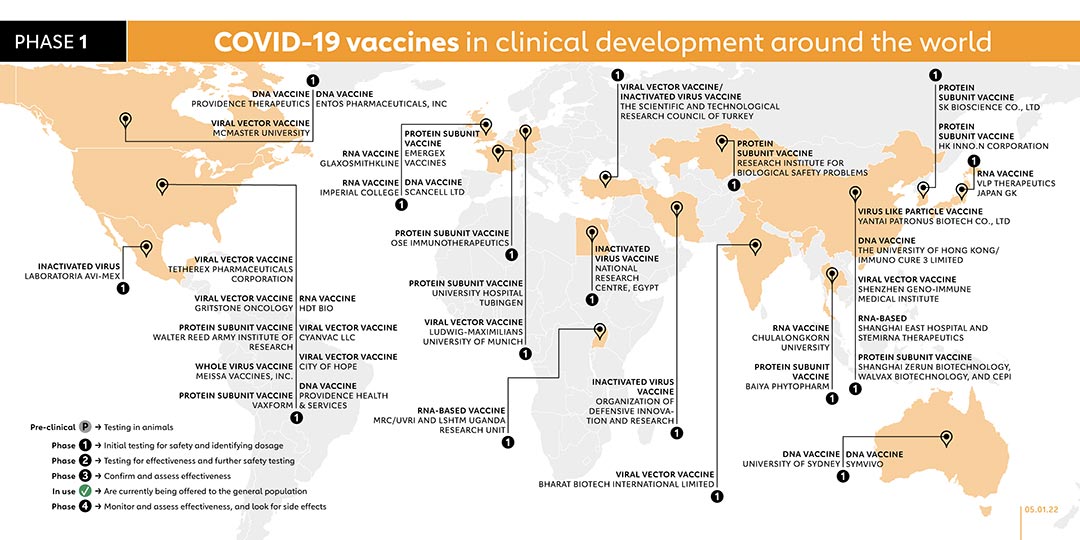
- 3
-
- CANSINO BIOLOGICS INC. (CHINA)
The company registered a phase 3 trial which is planned to be started in December 2021. The study aims to evaluate the efficacy and safety of the new investigational vaccine called Ad5-nCoV-IH which is administered through nebulized inhalation. The company registered a phase 3 trial in December 2021 to evaluate the safety and immunogenicity of a 2-dose regimen with CanSino's intramuscular Ad5-nCoV and inhaled Ad5-nCoV-IH vaccine in children and adolescents aged 6-17 Years. A total of 2000 participants will be equally divided into the two study countries, Chile and Mexico. All participants will receive a first dose of intramuscular Ad5-nCoV and a second dose of inhaled Ad5-nCoV-IH.
- GUANGDONG PROVINCIAL CENTER FOR DISEASE CONTROL AND PREVENTION (CHINA)
PROTEIN SUBUNIT VACCINE
In April 2021, Guangdong Provincial Center for Disease Control and Prevention and the Gaozhou Municipal Center for Disease Control and Prevention launched a phase 2 trial for its fusion protein COVID-19 vaccine, known as V-01. A phase 1 study began on 7 August 2021 with plans to recruit 43 participants. The study aims to evaluate the immunogenicity and safety of a third dose (booster dose). A phase 3 trial was started on 25 August 2021 to evaluate the efficacy, safety and immunogenicity of the company's vaccine. Another phase 3 trial was due to be carried out in October to evaluate the effectiveness of the company's vaccine as a booster dose for participants who have received two doses of inactivated vaccines.
- CODAGENIX/SERUM INSTITUTE OF INDIA (INDIA)
LIVE ATTENUATED VACCINE
Phase 1 trials of this intranasal vaccine, named COVI-VAC, started in the first week of January. As with the other nasal vaccines being trialled, it doesn’t require an injection nor does it need ultra-cold storage like some RNA vaccines. The World Health Organisation has included COVI-VAC as part of the candidate vaccines for its phase 3 trial.
- MEDICAGO (CANADA)
PLANT-BASED VIRUS-LIKE-PARTICLE VACCINE
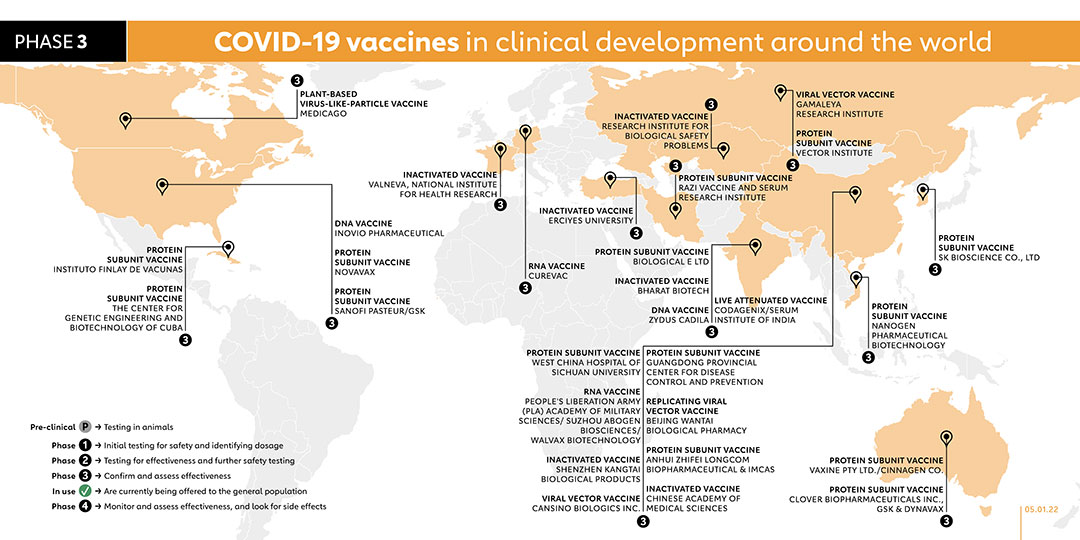
Partly funded by the cigarette manufacturer Philip Morris, Medicago uses a species of tobacco to make vaccines. Virus genes are delivered into leaves, and then plant cells create proteins that mimic those found on viruses. In July 2020 Medicago launched phase 1 trials on a plant-based COVID-19 vaccine in combination with adjuvants from GSK and Dynavax. Phase 2/3 trails started in November 2020 and the company plans to seek regulatory approvals in 2021 and the company plans to seek regulatory approvals in 2021. In October 2021, Medicago began recruiting 145 Japanese adults for phase 2 trial. A phase 3 trial was registered on 10 September 2021 to evaluate the safety and immunogenicity of the vaccine. The study is due to begin in November 2021. On 7 December 2021, Medicago and GlaxoSmithKline plc (GSK) announced positive efficacy and safety results from the global phase 3 study of Medicago’s plant-based COVID-19 vaccine candidate in combination with GSK’s pandemic adjuvant.
- BIOLOGICAL E LTD (INDIA)
PROTEIN SUBUNIT VACCINE
The company launched phase 1/2 trials in India in November 2020 for its COVID-19 vaccine, known as BECOV2, which is administered intramuscularly in a two-dose schedule. On 31 August 2021, it proceeded to phase 3.
- BHARAT BIOTECH INTERNATIONAL LTD (INDIA)
INACTIVATED VACCINE
In collaboration with the Indian Council of Medical Research and the National Institute of Virology, Bharat Biotech designed a vaccine called Covaxin based on an inactivated form of the coronavirus. On 3 Jan 2021, the Indian government granted Covaxin emergency authorization. Some countries in Africa, Asia, and South America have also authorised the vaccine. On 2 July 2021, promising results from a phase 3 clinical trial in 25 Indian hospitals were released.
- GAMALEYA RESEARCH INSTITUTE (RUSSIA)
Gamaleya has started a phase 1 clinical trial on this non-replicating viral vector vaccine candidate (known as Sputnik V) with two sets of volunteers receiving vaccination since mid-June. Before the vaccine went on to later trials Russia announced that it would be approved for use. A phase 3 trial of the vaccine began with more than 2,000 people in Russia, Latin America and the Middle East, and then expanded to 40,000. Volunteers were also recruited in Belarus, the UAE and Venezuela. On 2 February, the company published the results of their phase 3 trial. A study published in July found that Sputnik antibodies can neutralise the Delta variant, although not as effectively as with the original virus.
- NOVAVAX (USA)
PROTEIN SUBUNIT VACCINE
Novavax is using a nanoparticle technology platform to generate antigens from the spike protein found on the outer shell of the coronavirus. The vaccine, NVX-CoV2373, also received the highest funding from CEPI with a total of US$ 388 million and hundreds of millions of doses will be made available to the COVAX initiative if trials are successful. Phase 2 trials started in August in South Africa and phase 3 trials started in the UK in September as well as in the USA in December 2020.
In January 2021, the company announced that its vaccine had an efficacy rate of 89.3% based on trials in the United Kingdom. In South Africa, Novavax’s vaccine efficacy rate was lower at below 50%, potentially due to the dominance of the COVID-19 variant in South Africa, which can evade the antibodies stimulated by the vaccine. In both cases the vaccine was found to be 100% effective against severe disease. NVX-CoV2373 is among the vaccines within the COVAX portfolio. In November 2021, the company registered a phase 2 trial to evaluate the safety and immunogenicity of the company's vaccine in people living with HIV. A phase 2 trial study report was published in December 2021. The trial studied the safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of AstraZeneca's ChAdOx1 vaccine or Pfizer's BNT162b2 in the UK.
- ANHUI ZHIFEI LONGCOM BIOPHARMACEUTICAL & INSTITUTE OF MICROBIOLOGY, CHINESE ACADEMY OF SCIENCES (CHINA)
PROTEIN SUBUNIT VACCINE
The protein subunit vaccine candidate is being tested in collaboration with the Second Affiliated Hospital of Chongqing Medical University and Beijing Chao Yang Hospital. Phase 3 trials are being conducted across China. In July, a phase 1 clinical trial of the vaccine in people aged 3 to 17 was carried out. The company started a phase 3 clinical trial on 9 September 2021 to evaluate the consistency and immunogenicity of three doses of recombinant vaccine (CHO cells). On 4 November 2021, the company started a phase 2 clinical trial to compare the immunogenicity of recombinant new Coronavirus vaccine (CHO cells) among people aged 3 to 17 and 18 to 59 years of age. A phase 3 trial carried out by the company started on 17 September 2021. The trial uses mammalian CHO cells to evaluate the immunogenicity and safety of recombinant COVID-19 vaccine in adults.
- ZYDUS CADILA (INDIA)
DNA VACCINE
On 3 July Zydus Cadila announced approval to start human phase 1/2 trials, making it the second company in India to enter the COVID-19 vaccine race after Bharat Biotech. Phase 2 trials launched in August 2020. Zydus Cadila is now initiating a phase 3 trial with 300,000 participants.
- CHINESE ACADEMY OF MEDICAL SCIENCES (CHINA)
INACTIVATED VACCINE
The Institute of Medical Biology at the Chinese Academy of Medical Sciences (IMBCAMS) has previously developed inactivated vaccines for polio and hand-foot-and-mouth disease. Phase 2 trials began in July 2020 and was followed by a phase 3 trial in 29,000 participants in December 2020. On 5 September 2021, a phase 3 trial on sequential immunisation of recombinant COVID-19 vaccine (CHO cells) and inactivated COVID-19 vaccine (Vero Cells) was registered. Two phase 3 trials were registered in December 2021 to evaluate the immunogenicity and safety of the third dose SARS-CoV-2 Vaccine, inactivated (Vero Cell) in adults who inoculated the third dose after 3, 4, 5, or 6 months since finished two doses schedule. One of the trials will evaluate vaccines of Sinovac's CoronaVac or Sinopharm's BBIBP-CorV, while the other trial will evaluate vaccines of Institute of Medical Biology Chinese Academy of Medical Sciences.
- RESEARCH INSTITUTE FOR BIOLOGICAL SAFETY PROBLEMS (KAZAKHSTAN)
INACTIVATED VACCINE
Kazakhstan began research on a vaccine made from inactivated coronaviruses over the Summer. On 1 September 2020 it started phase 2 trials of its vaccine known as QazVac. On 19 December, it reported that phase 2 trials had been completed, with participants producing an immune response. In April 2021, Kazakhstan began administering the vaccine to the general public. On 29 July, government officials announced that 25,000 doses of the vaccine will be delivered to Kyrgyzstan. The phase 3 trial was completed in July 2021.
- SANOFI PASTEUR/GSK (FRANCE)
PROTEIN SUBUNIT VACCINE
The vaccine is based on the same design Sanofi used to create Flublok, an approved vaccine for influenza. The two companies launched a phase 1/2 clinical trial in September 2020. Following the announcement on 11 December 2020 that older people were not responding as strongly as expected to the vaccine, the companies began phase 2 trials in February 2021 with a different formulation of their vaccine and if successful, this study will support the selection of the most appropriate antigen dosage for phase 3. Phase 3 is projected to begin in the second quarter of 2021 if trial data is positive. Sanofi has agreed to provide much of its global supply to COVAX, an international collaboration co-led by Gavi to deliver the vaccine equitably across the world. In December, Sanofi said it has plans to make up to one billion doses in 2021. On 20 July 2021 the European Medicines Agency started a rolling review of the vaccine. Sanofi’s vaccine is among the vaccines in the COVAX portfolio. A phase 3 trial was registered in November 2021. The objective of this trial is to evaluate the immunogenicity and safety induced by a homologous vaccine booster (Pfizer-BioNTech vaccine booster) and a heterologous vaccine booster (one of the two experimental Sanofi/GSK vaccines booster), on the D614 (Wuhan) strain and on the SARS-CoV-2 variants, in adults who received 2 doses of Pfizer-BioNTech mRNA vaccine.
- INSTITUTO FINLAY DE VACUNAS (CUBA)
PROTEIN SUBUNIT VACCINE
In October 2020, Instituto Finlay De Vacunas launched trials on its second COVID-19 vaccine, Soberana 2. In December 2020, it launched a phase 2 trial and reached an agreement with the Pasteur Institute of Iran in January 2021 to test the vaccine in phase 3 trials. In March, a phase 3 trial was registered for Soberana 2. The vaccine has been used in Cuba and authorised for emergency use in Iran. A phase 3 study published in November 2021 proved that the company’s SOBERANA 02 vaccine is safe and has high efficacy in the adult population, while incorporating SOBERANA Plus could further increase efficacy.
- VECTOR INSTITUTE (RUSSIA)
PROTEIN SUBUNIT VACCINE
On 26 August, a Russian biological research centre known as the Vector Institute registered a phase 1/2 trial for a coronavirus vaccine called EpiVacCorona. On 14 October 2020, Vladimir Putin announced that Russia had granted regulatory approval for this vaccine, even though phase 3 trials had not yet begun. This was the second COVID-19 vaccine to be approved in Russia so far. The Vector Institute has since launched a phase 3 trial.
- THE CENTER FOR GENETIC ENGINEERING AND BIOTECHNOLOGY OF CUBA (CUBA)
PROTEIN SUBUNIT VACCINE
Along with its nasal spray vaccine, the Center for Genetic Engineering and Biotechnology of Cuba also launched a separate phase 1/2 trial in November 2020 for a vaccine called Abdala, which is injected into the muscle. In February 2021, the Center launched phase 2 trials for the Abdala vaccine, and in March a phase 3 trial, with a planned recruitment of more than 40,000 participants. The vaccine is authorised for emergency use in Cuba and Venezuela. A study report of a phase 1/2 trial was published in December 2021. The trial evaluated the safety and immunogenicity of the Abdala vaccine administered in different strengths and vaccination schedules. It found that the vaccine against SARS-CoV-2 was safe, well tolerated and induced immune responses against SARS-CoV-2 in adults.
- PEOPLE'S LIBERATION ARMY (PLA) ACADEMY OF MILITARY SCIENCES/ SUZHOU ABOGEN BIOSCIENCES/WALVAX BIOTECHNOLOGY (CHINA)
RNA VACCINE
Researchers at the Academy of Military Medical Sciences, Suzhou Abogen Biosciences and Walvax Biotechnology launched a phase 3 trial for their mRNA-based vaccine, called ARCoV, in April 2021. 28,000 volunteers are expected to participate.
- SHENZHEN KANGTAI BIOLOGICAL PRODUCTS/ BEIJING MINHAI BIOTECHNOLOGY CO., LTD. (CHINA)
INACTIVATED VACCINE
The Chinese manufacturing partner of AstraZeneca, Shenzhen Kangtai Biological Products, has its own inactivated COVID-19 vaccine known as Vero Cells. In April 2021 the company registered a phase 3 trial.
- VALNEVA, NATIONAL INSTITUTE FOR HEALTH RESEARCH (FRANCE/UK)
INACTIVATED VACCINE
Valneva launched a phase 1/2 trial in the UK in December 2020 for its inactivated, adjuvanted COVID-19 vaccine candidate, VLA2001. After announcing that the trial had delivered positive results, the company launched a phase 3 trial with 4,000 participants in the United Kingdom. Unlike current trials for COVID-19 vaccines, in which volunteers either receive the vaccine or a placebo, Valneva’s phase 3 trial, called “Cov-Compare,” will compare Valneva’s VLA2001 against AstraZeneca’s approved vaccine, Vaxzevria, in a comparative immunogenicity trial. The 4,000 participants will be equally split, with each half receiving two doses of either vaccine in order to allow researchers observe whether VLA2001 produces similar levels of antibodies to Vaxzevria.
- ERCIYES UNIVERSITY (TURKEY)
INACTIVATED VACCINE
Turkey-based Erciyes University completed a phase 1 trial for its vaccine candidate, called ERUCOV-VAC, in December 2020, which was followed by a phase 2 trial. In June 2021, the vaccine, now renamed TURKOVAC, begun phase 3 trials with over 40,000 participants. Its efficacy is being compared with CoronaVac (the vaccine from Sinovac). In July, a phase 2 trial was carried out to evaluate the vaccine as a booster shot. A phase 3 trial began on 8 October to evaluate the vaccine as a booster shot.
- WEST CHINA HOSPITAL OF SICHUAN UNIVERSITY (CHINA)
PROTEIN SUBUNIT VACCINE
The vaccine incorporates recombinant protein grown in insect cells. To make the vaccine, researchers encode the receptor-binding domain (RBD) in a gene, which they then insert into a virus. They then infect insect cells with the virus, triggering them to make the molecule in huge amounts. The hospital’s State Key Laboratory of Biological Therapy developed the “insect vaccine,” which seems to stop infection in monkeys without showing any apparent side effects. In May 2021, a phase 3 trial was launched with 40,000 participants.
- NANOGEN PHARMACEUTICAL BIOTECHNOLOGY (VIETNAM)
PROTEIN SUBUNIT VACCINE
Vietnam-based Nanogen launched its phase 1/2 trial for its vaccine, known as nanocovax, with 620 participants in December 2020. In June 2021, the company registered a phase 3 trial with 13,000 participants.
- VAXINE PTY LTD./CINNAGEN CO. (AUSTRALIA/IRAN)
PROTEIN SUBUNIT VACCINE
Vaxine launched a phase 1 trial in July 2020. Its vaccine, called SpikoGen, combines viral proteins with an adjuvant that stimulates immune cells. In May 2021, the company registered a phase 2 trial in Iran with vaccine makers, CinnaGen Co, followed by a phase 3 trial registered in August 2021 to evaluate efficacy and safety of the vaccine. In December 2021, the company registered a phase 2/3 trial to assess effectiveness of SpikoGen Covid-19 Vaccine. SpikoGen has been authorised by Iran for emergency use.
- SK BIOSCIENCE CO., LTD (SOUTH KOREA)
PROTEIN SUBUNIT VACCINE
In February 2021, SK Bioscience launched a phase 1/2 trial, involving more than 200 healthy adults, for its COVID-19 vaccine candidate. The vaccine, called GBP510, is administered in two doses. A phase 3 trial was registered in August 2021, with plans to recruit 3990 volunteers. A phase 2 trial was registered in January 2022 to assess safety, reactogenicity and immunogenicity of booster vaccination of the company's SARS-CoV-2 vaccine called GBP510 in adults who received a primary series of vaccination against COVID-19 approved in Korea.
- CLOVER BIOPHARMACEUTICALS INC., GSK & DYNAVAX (AUSTRALIA)
PROTEIN SUBUNIT VACCINE
The protein-based COVID-19 S-Trimer vaccine, which uses GSK’s adjuvant system, is now being tested in human clinical trials. Clover launched phase 1 trials in June 2020, after obtaining US$ 3.5 million from Coalition for Epidemic Preparedness Innovations (CEPI). After announcing that phase 1 trial participants produced a high level of antibodies, Clover began phase 2/3 trials with the GSK adjuvant in December 2020. A phase 3 trial was registered in August. Clover is among the vaccines in the COVAX portfolio. A phase 2 trial was registered on 21 October 2021 to study the immunogenicity and safety of heterologous and homologous boosting with AstraZeneca/University of Oxford's ChAdOx1-S and Sinovac-CoronaVac or a formulation of Clover's SCB-2019. The trial expects to enrol 520 participants and is due to be conducted on 1 December 2021.
- RAZI VACCINE AND SERUM RESEARCH INSTITUTE (IRAN)
PROTEIN SUBUNIT VACCINE
The Razi Vaccine and Serum Research Institute vaccine, known as Razi Cov-Pars, contains coronavirus-like spike proteins and is delivered in three doses via two injections and one nasal spray. According to the company, the vaccine is the first injectable-inhaled COVID-19 vaccine of recombinant protein. In April 2021, a phase 2 trial was launched for Razi Cov-Pars, with 500 volunteers participating. On 29 August 2021, the company registered a phase 3 trial with a planned recruitment of more than 41,000 participants. A trial was registered on 29 November 2021 to compare the immunogenicity and safety of Razi Cov Pars and Sinopharm booster doses in adults who have primarily vaccinated with Sinopharm.
- BEIJING WANTAI BIOLOGICAL PHARMACY (CHINA)
In 2019, researchers at the University of Hong Kong and Xiamen University created a nasal-spray vaccine for the flu based on a weakened form of the influenza virus. Now they have engineered the vaccine to produce part of the coronavirus spike protein, and have started trials in partnership with Beijing Wantai Biological Pharmacy. A phase 2 study with 720 participants started in November 2020 in China’s eastern province of Jiangsu. The vaccine then moved into trials in September 2021.
- INOVIO PHARMACEUTICAL (USA)
DNA VACCINE
Inovio put its INO-4800 DNA vaccine into human trials early in April 2020. So far, it is the only company with a phase 2/3 vaccine against the related MERS-CoV coronavirus. The company has begun human clinical trials in the USA, China and South Korea. INO-4800 DNA has been included in the WHO phase 3 trial.
- NATIONAL VACCINE AND SERUM INSTITUTE, CHINA (CHINA)
PROTEIN SUBUNIT VACCINE
Beijing-based National Vaccine and Serum Institute launched a phase 1/2 trial for its COVID-19 vaccine candidate, known as CHO Cell with over 3,000 participants aged 3 years and older. The clinical trial will cover 5 age groups: 3-8 years old, 9-17 years old, 18-59 years old, 60-69 years old, and those aged 70 and over. CHO Cell will be administered as a two-dose vaccine. In October 2021, CHO Cell has registered and began recruiting 1,848 participants for their phase 3 trial with an estimated study conclusion date of February 2024.
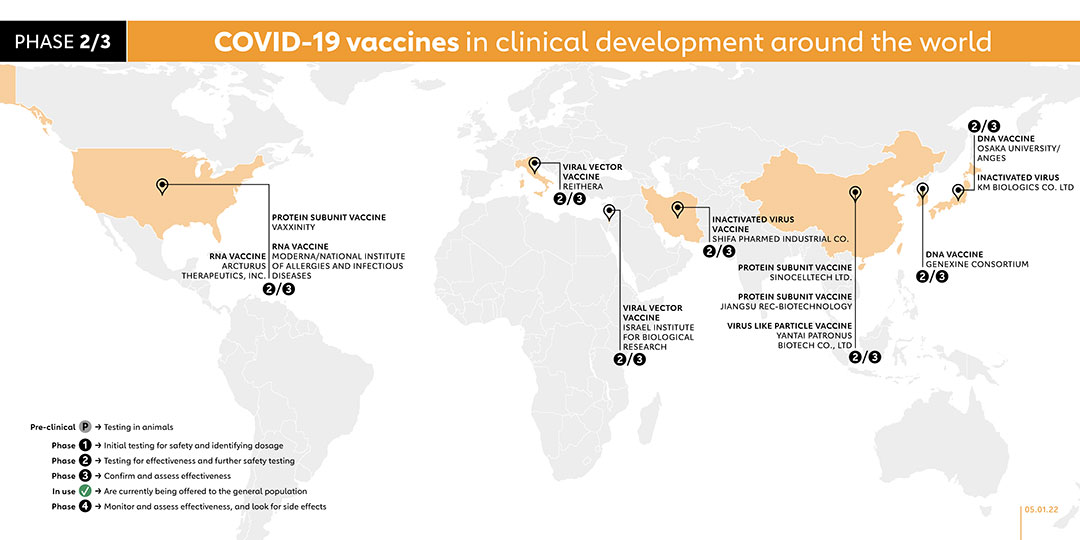
- 2/3
-
- YANTAI PATRONUS BIOTECH CO., LTD (CHINA)
Yantai Patronus Biotech Company has developed a virus-like particle vaccine called LYB001. A phase 1 trial was registered in November 2021 to evaluate the safety and immunogenicity of the vaccine. The company registered a phase 2/3 trial to evaluate the immunogenicity and safety profile of LYB001 in adults, administered with a three-dose regimen with 28 days interval.
- KM BIOLOGICS CO. LTD (JAPAN)
INACTIVATED VIRUS
KM Biologics registered a phase 1/2 trial for its inactivated COVID-19 vaccine candidate, known as KD-414. The trial for the two-dose vaccine will involve adult participants aged 20 years and above. A phase 2/3 trial was registered on 22 October 2021 to evaluate the safety and immunogenicity of two doses of the company's COVID-19 vaccine - KD-414 - in healthy Japanese subjects aged 18 years or older.
- JIANGSU REC-BIOTECHNOLOGY (CHINA)
PROTEIN SUBUNIT VACCINE
Taizhou-based Jiangsu Rec-Biotechnology launched a phase 1 trial for its vaccine, known as ReCOV. The vaccine candidate is a recombinant two-component COVID-19 vaccine (CHO cell), which adopts an advanced neutralising antibody-guided and structure-based vaccine design concept. Compared with whole virus inactivated vaccine or S full-length protein vaccine, it is expected to make the immune resources more focused and reduce the ADE risk caused by non-neutralising antibodies. On 20 October 2021, the company registered a phase 2/3 trial to evaluate the safety and immunogenicity of a recombinant two-component COVID-19 vaccine (ReCOV) in adults, as well as the efficacy, safety, and immunogenicity of ReCOV. With plans to enrol 20,301 participants, the trial is due to be conducted from 31 December 2021.
- GENEXINE CONSORTIUM (SOUTH KOREA)
DNA VACCINE
This vaccine is being jointly developed by a consortium which includes Genexine, Binex, GenNBio, International Vaccine Institute, Korea Advanced Institute of Science, and Technology and Pohang University of Science and Technology. The vaccine is currently in clinical phase 1/2 with tests being conducted on 190 healthy participants between the age of 18 to 50. Genexine plans to market the vaccine by the second half of 2021. On 5 October 2021, the company registered a phase 2/3 trial. With plans to recruit 14,000 participants, the trial will evaluate the efficacy, safety and immunogenicity of the vaccine in healthy individuals who have received one dose of COVID-19 vaccine.
- SINOCELLTECH LTD. (CHINA)
PROTEIN SUBUNIT VACCINE
Chinese biotechnology company Sinocelltech registered a phase 2/3 trial for the vaccine called SCTV01C on 14 September 2021. With a plan to recruit 12,420 participants, the study aims to evaluate the vaccine's efficacy in healthy populations previously vaccinated with adenovirus vector or mRNA COVID-19 vaccines. The study is due to begin on 30 October 2021. The company started a phase 2/3 trial in December to evaluate the safety, tolerability and immunogenicity of the company's experimental vaccine called SCTV01C-02-1 in healthy adults who are previously unvaccinated.
- OSAKA UNIVERSITY/ANGES (JAPAN)
DNA VACCINE
On 30 June, the Japanese biotechnology company AnGes announced it had started safety trials (phase 1) on a DNA-based vaccine, developed in partnership with Osaka University and Takara Bio. They launched a phase 2/3 trial in December 2020.
- VAXXINITY (USA)
PROTEIN SUBUNIT VACCINE
COVAXX, a subsidiary of United Biomedical, registered a phase 1 trial on 11 September 2020 in Taiwan for its COVID-19 vaccine candidate, UB-612. It launched a phase 2/3 trial in f February 2021 and announced further plans to begin pre-clinical research on a vaccine specifically against newly emerging COVID-19 variants. In April, the company merged with United Neuroscience, under the newly formed holding company, Vaxxinity, in order to consolidate vaccine development efforts. UB-612 remains the lead vaccine candidate in the pipeline. A phase 1 trial was registered on 20 July 2021 to assess the effectiveness of a third dose of the vaccine.
- REITHERA (ITALY)
The Italian biotechnology company ReiThera has developed a COVID-19 vaccine, called GRAd-COV2, based on an adenovirus that infects gorillas. In collaboration with the Lazzaro Spallanzani National Institute for Infectious Diseases it launched a phase 1 trial at the end of July 2020, with subsequent results indicating that participants produced antibodies. The company has launched a phase 2/3 trial.
- SHIFA PHARMED INDUSTRIAL CO (IRAN)
INACTIVATED VACCINE
Iran-based Shifa Pharmed Industrial Co. created a vaccine called COVIran Barekat and launched a phase 2/3 trial with 20,000 volunteers in six cities including Tehran. A phase 1/2 trial was registered in November 2021 to compare the tolerability, safety, and immunogenicity of Shifa-Pharmed's vaccine (CovIran) and Sinopharm's vaccine in the healthy population aged 12 to 18 years.
- MODERNA/NATIONAL INSTITUTE OF ALLERGIES AND INFECTIOUS DISEASES (US)
RNA VACCINE
Moderna has begun a phase 2/3 trial to assess the efficacy of its vaccine, mRNA-1273.211, as a single booster dose against variants of concern for those who have previously received two doses of mRNA-1273, which is currently in phase 4 trials.
- ISRAEL INSTITUTE FOR BIOLOGICAL RESEARCH (ISRAEL)
Phase 2 trials of this vaccine have just begun with 1,000 participants. Phase 1 participants showed no side effects. It expects to finalise phase 3 trials in the summer of 2021. On 15 August 2021, its partner American company NRx Pharmaceuticals registered a phase 2/3 trial which targets to involve 550 participants.
- ARCTURUS THERAPEUTICS, INC. (US)
RNA VACCINE
A Phase 2/3 study was carried out in August 2021 to evaluate the safety, immunogenicity and efficacy of the ARCT-154 mRNA vaccine in adult participants to be enrolled in Vietnam. ARCT-154 mRNA vaccine was included in the WHO phase 3 trial in October 2021 to run concurrently with phase 2/3 trial.
On 30 August 2021, a phase 1/2 trial was carried out to evaluate the safety, reactogenicity, and immunogenicity of three vaccine candidates in parallel – the current ARCT-154 vaccine, and two new vaccines ARCT-165 and ARCT-021 – against COVID-19 in adults previously vaccinated and those who are not. The study involves approximately 72 adult participants and may expand enrolment up to 144 participants if additional cohorts are added. This study is due to be completed in March 2023.
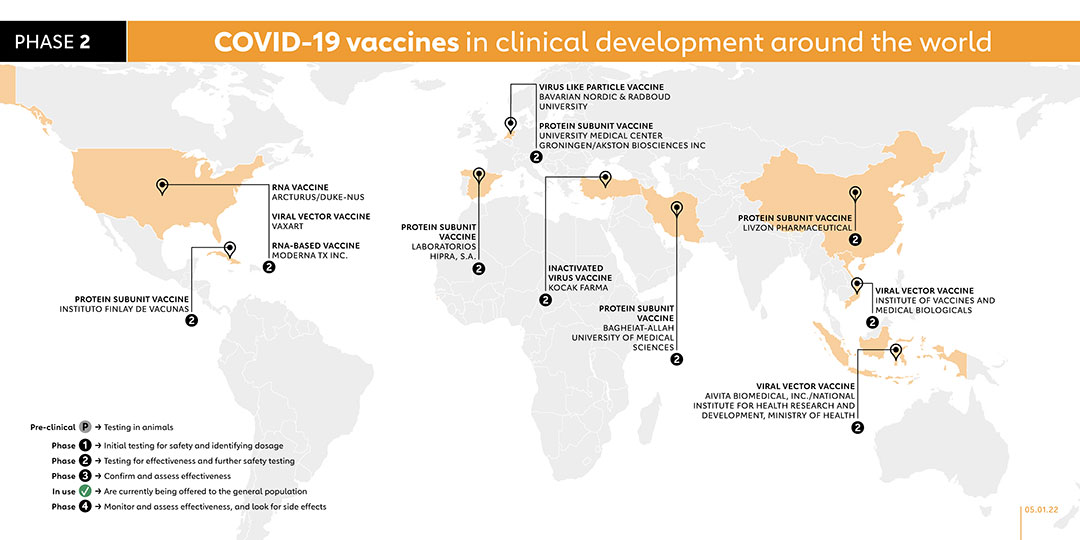
- 2
-
-
MODERNA TX INC. (US)
RNA VACCINE
Moderna has developed a third COVID-19 vaccine candidate: a refrigerator-stable mRNA vaccine that will facilitate easier distribution and administration by healthcare providers. The vaccine, known as mRNA-1283, will be administered alongside another Moderna vaccine, mRNA-1273, which has already been granted Emergency Use Listing by the World Health Organization and is currently being offered to the general population.
The phase 1 trial will evaluate the safety and efficacy of three different dose levels of the mRNA-1283 vaccine candidate given as a two-dose series, 28 days apart, and one dose level of mRNA-1283 given to healthy adults in a single dose. These will be compared with a two-dose series of mRNA-1273, the currently authorised dose level. The company expects mRNA-1283 to be evaluated in future studies for use both as a booster dose for previously vaccinated or seropositive individuals and in a primary series for seronegative individuals. In November 2021, the company registered a phase 2 trial to evaluate the immunogenicity and safety of the company's mRNA-1283 COVID-19 vaccine boosters to boost antibody responses to the ancestral strain of SARS-CoV-2 virus, the B.1.351 variant, and potentially other SARS-CoV-2 variants. In November 2021, the company registered a phase 2 study to evaluate the immunogenicity and safety of the company's mRNA-1283 COVID-19 vaccine boosters to boost antibody responses to the ancestral strain of SARS-CoV-2 virus, the B.1.351 variant, and potentially other SARS-CoV-2 variants.
- LIVZON PHARMACEUTICAL (CHINA)
PROTEIN SUBUNIT VACCINE
collaboration with the Institute of Biophysics at the Chinese Academy of Sciences. The company started a clinical study in September 2021 to evaluate the immunogenicity and safety of sequential immunisation of the company's protein subunit vaccine as a booster dose in healthy adults who have received two doses of inactivated vaccines.
- UNIVERSITY MEDICAL CENTER GRONINGEN/AKSTON BIOSCIENCES INC (THE NETHERLANDS)
PROTEIN SUBUNIT VACCINE
This vaccine is about to go into phase 1/2 trials with 130 adult participants in January 2021. A phase 2 trial was registered in November 2021 to investigate if a subcutaneous booster dose of the Akston's AKS-452 vaccine after initial vaccination with Pfizer, Moderna, Janssen or AstraZeneca will boost the antibody titer and immune response.
- LABORATORIOS HIPRA, S.A. (SPAIN)
Protein Subunit
In August 2021, Spain’s Laboratorios Hipra, S.A. commenced their phase 1/2 trials with 30 randomised healthy participants between the ages of 18-39 for their vaccine: COVID-19 Vaccine Hipra. A Phase 2 trial was registered in November 2021 to evaluate the safety and immunogenicity of the company's PHH-1V vaccine as a booster dose in adults already fully vaccinated against COVID-19.
- VAXART (USA)
Early in 2020, Vaxart began working on an oral vaccine for COVID-19 using an adenovirus called Ad5 to deliver part of the COVID-19 virus into the body to trigger an immune response. In October 2020 it began phase 1 trials. On 5 October 2021, the company registered a phase 2 trial. With plans to recruit 896 participants, the study will evaluate the safety, immunogenicity and efficacy of the vaccine. This study is due to be completed by June 2023.
- KOCAK FARMA (TURKEY)
INACTIVATED VACCINE
In April 2021, Koçak Farma launched a phase 1 trial for its COVID-19 vaccine candidate called Koçak-19 Inaktif Adjuvanlı, which contains inactivated coronavirus. A phase 2 trial was registered on 5 September 2021 for a novel COVID-19 vaccine candidate called TURKOVAC. The study will compare two different dose levels and immunisation regimen of the vaccine against SARS-CoV-2 infection. The aim is to assess the efficacy and safety of these vaccine strengths after three doses. This study will also provide more data to assess the efficacy of the third dose (booster dose) for COVID-19 prevention.
- ARCTURUS/DUKE-NUS (USA/SINGAPORE)
RNA VACCINE
Phase 2 trials of this vaccine have begun in January, 2021 in Singapore and the United States. Phase 1/2 participants produced an immune response similar to those who recover from COVID-19.
- INSTITUTO FINLAY DE VACUNAS (CUBA)
PROTEIN SUBUNIT VACCINE
The Finlay Vaccine Institute in Havana have developed a single-dose vaccine called Soberana Plus, which is its third candidate currently in clinical trials. Like its other candidates, Soberana 1 and Soberana 2, Soberana Plus contains a part of the spike protein, called RBD, along with adjuvants to boost an immune response to COVID-19. Unlike the other two, Soberana Plus is specifically targeted at people with a history of COVID-19 infection. The Institute’s Soberana 1 vaccine is currently registered for a phase 2 clinical trials with 450 participants.
- AIVITA BIOMEDICAL, INC./NATIONAL INSTITUTE FOR HEALTH RESEARCH AND DEVELOPMENT, MINISTRY OF HEALTH (INDONESIA)
AIVITA Biomedical completed a phase 2 trial with 145 participants in May 2021. In this phase 2 study, efficacy is assessed and safety is confirmed via laboratory values, observation and regular patient reporting.
- RADBOUD UNIVERSITY (THE NETHERLANDS)
A phase 1 trial was launched in April 2021 for Radboud University’s COVID-19 vaccine candidate known as ABNCoV2. Injected intramuscularly in two doses, ABNCoV2 is being tested as a formulation both with and without the adjuvant MF59 across 42 adult participants in order to identify the dosage and formulation that best optimises the vaccine’s efficacy 14 days following first vaccination. On 19 August 2021, Radboud University, under Bavarian Nordic, started a phase 2 trial to evaluate safety, tolerability and immunogenicity of the ABNCoV2 vaccine in seronegative and seropositive adults, as well as adults with a history of SARS-CoV-2 vaccination or previous COVID-19 disease three months prior to enrolment.
- BAGHEIAT-ALLAH UNIVERSITY OF MEDICAL SCIENCES (IRAN)
PROTEIN SUBUNIT VACCINE
On 25 June 2021, a phase 1 trial was registered for RBD protein recombinant SARS-CoV-2 vaccine. The trial is currently recruiting 70 participants between 18 and 50 years old. In October 2021, the vaccine was registered for a phase 2 trial on 300 volunteers.
-
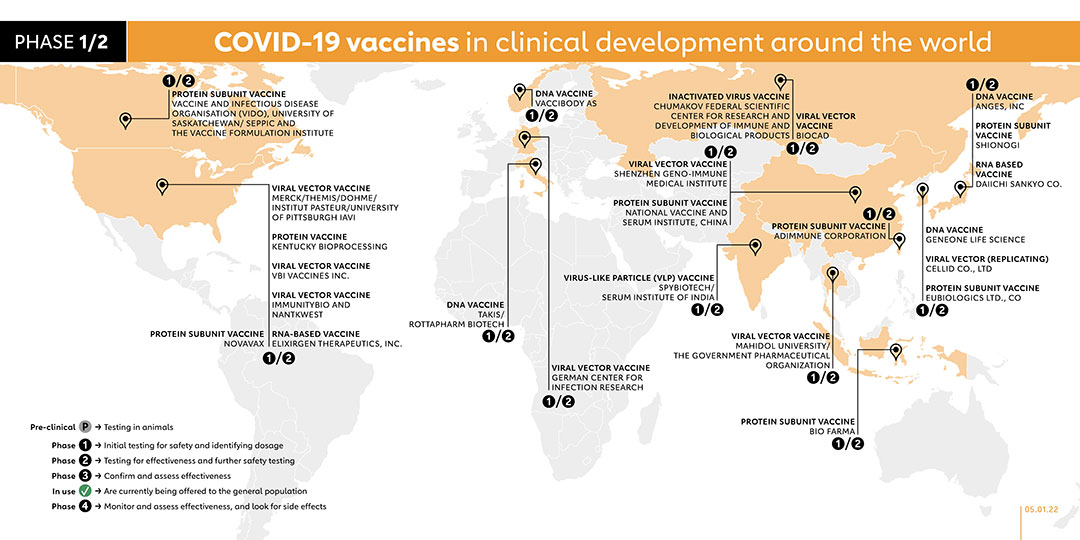
- 1/2
-
- ADIMMUNE CORPORATION (TAIWAN)
PROTEIN SUBUNIT VACCINE
Adimmune launched a phase 1 trial in August 2020 with 70 adult participants. A phase 1/2 study was registered in November 2021 to evaluate the vaccine safety and immunogenicity of the company’s AdimrSC-2f vaccine in healthy adult individuals, as compared to placebo.
- BIO FARMA (INDONESIA)
PROTEIN SUBUNIT VACCINE
PT Bio Farma in collaboration with Fakultas Kedokteran Universitas Indonesia National Institute of Health Research and Development and Ministry of Health Republic of Indonesia registered their vaccine for phase 1/ 2 trial in October 2021.
- VACCIBODY AS (NORWAY)
DNA VACCINE
Vaccibody AS, a Norwegian company, registered a phase 1/2 trial on 6 October 2021. The study aims to evaluate the safety, reactogenicity and immunogenicity of the company's two vaccine candidates, VB10.2129 (C1) and VB10.2210 (C2). With plans to recruit 160 participants, the study is due to be completed in October 2023.
- CHUMAKOV FEDERAL SCIENTIFIC CENTER FOR RESEARCH AND DEVELOPMENT OF IMMUNE AND BIOLOGICAL PRODUCTS (RUSSIA)
INACTIVATED VIRUS VACCINE
Chumakov’s new vaccine CoviVac is undergoing phase 1/ 2 clinical trials with 400 participants.
- BIOCAD (RUSSIA)
Biocad, a Russian biotechnology company, started a phase 1/2 trial for the BCD-250 vaccine on 10 August 2021. The vaccine uses an adenovirus-associated virus called AAV-5 as a vector which carries a gene encoding the SARS-CoV-2 spike protein. The study has a plan of enrolling 160 participants to assess the safety and immunogenicity of the vaccine for preventing COVID-19 infection. The study is due to be completed in December 2022.
- NOVAVAX (USA)
PROTEIN SUBUNIT VACCINE
On 1 September 2021, Novavax registered a phase 1/2 study to evaluate the safety and immunogenicity of three vaccines, namely SII B.1.351, SII Bivalent, and SII B.1.617.2 respectively, when adjuvanted with Novavax’s Matrix-M1 flu shot. The SII B.1.351 is a two dose vaccine 21 days apart, the SII Bivalent is on trial as a singular dose and the SII B.1.617.2 is on trial as a two dose vaccine 21 days apart. This study plans to involve 240 participants and is due to be conducted in October 2021.
- ANGES, INC (JAPAN)
DNA VACCINE
AnGes, Inc commenced phase 1/2 trials on its vaccine, AG0302-COVID19, with 400 randomized participants aged 18 years or older in August 2021.
- KENTUCKY BIOPROCESSING (USA)
PROTEIN SUBUNIT VACCINE
Similar to Medicago, this second tobacco-based vaccine is created by engineering a species of tobacco called Nicotiana benthamiana to make viral proteins. Kentucky Bioprocessing used this technique to make a drug called Zmapp for Ebola. After preclinical testing in the Spring in 2020, it registered a phase 1/2 trial for its coronavirus vaccine in July and launched the trial in December.
- SPYBIOTECH/SERUM INSTITUTE OF INDIA (INDIA)
VIRUS-LIKE PARTICLE (VLP) VACCINE
This spin-off from Oxford University started its phase 1/2 trial in Australia, run by the Serum Institute of India. Human papillomavirus (HPV) vaccines and some hepatitis B vaccines are also based on the VLP-platform.
- VACCINE AND INFECTIOUS DISEASE ORGANISATION (VIDO), UNIVERSITY OF SASKATCHEWAN/ SEPPIC AND THE VACCINE FORMULATION INSTITUTE (VFI)(CANADA)
PROTEIN SUBUNIT VACCINE
VIDO’s vaccines have just entered phase 1/2 trials. It expects at least one of its candidates to be ready by late 2021.
- GENEONE LIFE SCIENCE (SOUTH KOREA)
DNA VACCINE
This South Korean biotech company developed a DNA-based vaccine and launched a phase 1/2 trial with 345 participants in December 2020. The company registered a phase 1 trial on 20 October 2021 to study the safety, tolerability and immunogenicity of their DNA vaccine against SARS-CoV-2. With plans to enrol 30 participants, the study is due to be conducted in November 2021.
- SHIONOGI (JAPAN)
PROTEIN SUBUNIT VACCINE
The company registered a phase 1/2 trial with around 200 Japanese adult participants in December 2020.
- CELLID CO., LTD (SOUTH KOREA)
In April 2020, Cellid began to develop a vaccine for COVID-19. After the vaccine was tested out on monkeys, Cellid partnered with South Korean chemical manufacturer, LG Chem, to manufacture the vaccines. In December 2020, they registered a phase 1/2 trial with over 100 participants. On 9 September 2021, a phase 1 clinical trial began to assessvaccine safety and immune responses against SARS-CoV-2.
- THE CENTER FOR GENETIC ENGINEERING AND BIOTECHNOLOGY OF CUBA (CUBA)
PROTEIN SUBUNIT VACCINE
The Center for Genetic Engineering and Biotechnology of Cuba launched its phase 1/2 clinical trials in November 2020. Its nasal spray COVID-19 vaccine is called Mambisa and contains a piece of the coronavirus spike protein as well as a protein from the hepatitis B virus, which stimulates the immune system.
- SHENZHEN GENO-IMMUNE MEDICAL INSTITUTE (CHINA)
In addition to a phase 1 trial for its COVID-19 aAPC vaccine, the Shenzhen Geno-Immune Medical Institute has launched a separate phase 1/2 trial for its vaccine called LV-SMENP-DC vaccine, with 100 participants.f
- TAKIS AND ROTTAPHARM BIOTECH (ITALY)
DNA VACCINE
Takis and Rottapharm Biotech jointly developed a vaccine known as COVID-eVax. The vaccine is administered by an intramuscular injection, followed by electrical pulses to the skin in a procedure known as Electro-Gene-Transfer (EGT) which assists with delivery of the vaccine into the cells. The companies launched a phase 1/2 trial in February 2021.
- MAHIDOL UNIVERSITY/THE GOVERNMENT PHARMACEUTICAL ORGANIZATION (THAILAND)
In close collaboration with the Thai Government Pharmaceutical Organization, Mahidol University launched a phase 1/2 trial for a COVID-19 vaccine called NDV-HXP-S, which was developed by Icahn School of Medicine at Mount Sinai in New York. NDV-HXP-S is based on the Newcastle Disease Virus (NDV), which can be grown in large quantities in chicken eggs, in a similar way to the well-established influenza vaccines that have been produced since the 1950s.
- VBI VACCINES INC (USA)
Massachusetts-based VBI Vaccines Inc launched a phase 1/2 trial for its vaccine candidate, VBI-2902a, with over 700 participants in Canada. The trial will compare the effects of using one or two doses of VBI-2902a, which is designed to induce neutralising antibody and cell-mediated immune responses against the SARS-CoV-2 spike protein.
- EUBIOLOGICS LTD., CO (SOUTH KOREA)
PROTEIN SUBUNIT VACCINE
EuBiologics launched a Phase 1/2 trial of for its recombinant protein-based vaccine, known as EuCorVac-19, with over 200 participants.
- DAIICHI SANKYO CO.
RNA VACCINE
In collaboration with the University of Tokyo, Daiichi Sankyo Co. launched a phase 1/2 trial for its mRNA vaccine candidate, known as DS-5670a, in March 2021.
- INSTITUTE OF VACCINES AND MEDICAL BIOLOGICALS (VIETNAM)
The Institute of Vaccines and Medical Biologicals launched a phase 1/2 trial with more than 400 participants for its COVID-19 vaccine candidate known as COVIVAC.
- IMMUNITYBIO AND NANTKWEST (USA)
The company launched a phase 1 trial in California in December with people aged up to 55 years. It is an adenovirus (Ad5) vaccine designed to deliver both spike protein and nucleocapsid DNA to drive both cellular and antibody immunity. In 2020, it was chosen to be part of Operation Warp Speed to rapidly develop vaccines in the USA. In April 2021, a phase 1/2 trial was launched with 540 participants.
- ELIXIRGEN THERAPEUTICS, INC. (US)
RNA VACCINE
In April 2021, Baltimore-based Elixirgen Therapeutics launched a phase 1/2 trial for its self-replicating RNA-based COVID-19 vaccine called EXG-5003. The vaccine, administered in a single dose, is temperature-sensitive and expresses the receptor binding domain of the coronavirus spike protein.
- GERMAN CENTER FOR INFECTION RESEARCH (GERMANY)
Over thirty years ago, the German Center for Infection Research developed a smallpox vaccine from a harmless virus known as Modified Vaccinia Ankara (MVA). This was subsequently adapted to create a vaccine for MERS, a disease caused by another coronavirus. The center has now developed an MVA-based vaccine for COVID-19. The vaccine contains the MVA vector expressing a stabilised SARS-CoV-2 spike protein (S), which is produced inside cells that it invades. In May 2021, it launched a phase 1/2 trial with 240 adult participants.
- RESEARCH INSTITUTE FOR BIOLOGICAL SAFETY PROBLEMS (KAZAKHSTAN)
PROTEIN SUBUNIT VACCINE
In June 2021, the Research Institute for Biological Safety Problems launched a phase 1/2 trial for its vaccine, known as QazCoVac-P, with over 200 adult participants. QazCoVac-P is the second COVID-19 vaccine developed by the Research Institute. Unlike the first vaccine, QazVac, which is made from inactivated viruses, QazCoVac-P uses proteins from the virus to stimulate an immune response.
- 1
-
- VLP THERAPEUTICS JAPAN GK (JAPAN)
RNA VACCINE
VLP Therapeutics Japan GK, a Japanese company, has developed a self-amplifying RNA vaccine called VLPCOV-01 and registered a phase 1 trial in September 2021.
- HDT BIO (USA)
RNA VACCINE
HDT Bio developed a RNA based vaccine called HDT-301 in collaboration with the National Institutes of Health Rocky Mountain Laboratories, HDT Bio Corp, and Gennova Biopharmaceuticals. In November 2021, a phase 1 trial was registered to assess tolerability and immunogenicity of three dose levels of the company's investigational vaccine both in unimmunised participants and as a booster for those participants who previously received a SARS-CoV-2 vaccine.
- NATIONAL RESEARCH CENTRE, EGYPT (EGYPT)
INACTIVATED VIRUS VACCINE
Egypt’s National Research Centre has developed an inactivated coronavirus vaccine called Covi Vax. The researchers registered a phase 1 trial in November 2021. The study aims to test the vaccine’s effectiveness at different doses.
- HK INNO.N CORPORATION (KOREA)
PROTEIN SUBUNIT VACCINE
The Korea biotechnology company HK inno.N has developed a recombinant protein vaccine against the SARS-CoV-2. The company began a phase 1 trial on 16 September 2021 to evaluate the safety and immunogenicity of their vaccine called IN-B009 in healthy adults.
- EMERGEX VACCINES (BRITAIN)
PROTEIN SUBUNIT VACCINE
On 9 November 2021, the British company Emergex Vaccines has registered a phase 1 trial to investigate the safety of their COVID-19 vaccine. The vaccine is repurposed from the company’s Dengue vaccine platform which could stimulate the immune system against SARS-CoV-2 without antibodies.
- THE UNIVERSITY OF HONG KONG/IMMUNO CURE 3 LIMITED (HONG KONG)
DNA VACCINE
Researchers at the University of Hong Kong registered a phase 1 trial on 1 November 2021 to test a DNA vaccine against the coronavirus. The trial will deliver a dose into the participants’ muscles with the use of high-voltage electric shocks to induce cells into receiving the vaccine, potentially improving vaccine uptake.
- MCMASTER UNIVERSITY (CANADA)
A phase 1 trial was registered in October 2021, with plans to recruit 30 participants who have received two doses of an mRNA COVID-19 vaccine. This study is due to be conducted from 29 November 2021. It will evaluate the safety and immune responses following the administration by aerosol of either the Ad5-triCoV/Mac or ChAd-triCoV/Mac vaccine. These are new experimental adenovirus-based vaccines, delivered through inhalation, were developed by the university in collaboration with the Canadian Institutes of Health Research.
- YISHENG BIOPHARMA (CHINA)
PROTEIN SUBUNIT VACCINE
In August 2021, Yisheng Biopharma registered their vaccine for phase 1 clinical trial, with the aim to enrol 45 participants in their study.
- SCANCELL LTD (UK)
DNA VACCINE
Scancell Ltd registered its vaccine Covidity for phase 1 trials in September 2021 and expects to enrol 40 participants.
- PROVIDENCE HEALTH & SERVICES (USA)
DNA VACCINE
Phase 1 trials in healthy participants started recruiting participants in December 2020, enrolling 36 volunteers in the initial phase.
- LUDWIG-MAXIMILIANS UNIVERSITY OF MUNICH (GERMANY)
On 30 September, phase 1 testing for this non-replicating viral vector vaccine was approved, involving 30 healthy participants aged between 18 and 55 years.
- CITY OF HOPE (USA)
Phase 1 trials of this vaccine based on a weakened form of a virus called Modified Vaccinia Ankara, or MVA for short, began on 24 November, and started phase 2 in July 2021.
- UNIVERSITY HOSPITAL TÜBINGEN (GERMANY)
PROTEIN SUBUNIT VACCINE
Earlier this year, researchers at the University of Tubingen in Germany created a vaccine made of viral proteins, along with an immune-stimulating adjuvant. In September 2020, it launched a phase 1 trial. In June 2021, Phase 1/2 trials were launched with 68 participants.
- IMPERIAL COLLEGE (UK)
RNA VACCINE
This vaccine candidate which uses self-amplifying RNA technology (saRNA) has been developed by researchers at Imperial College London. The research has received £ 41 million in funding from the UK government. It has also received £ 5 million through philanthropic donations. The clinical trial in the current phase will involve administration of the two-dose vaccine to 300 healthy individuals. Volunteers have already started receiving the first dose of the vaccine. Imperial College has also created VacEquity Global Health, a social enterprise, to develop and distribute low-cost vaccines around the world, including to low- and middle-income countries. It began phase 1/2 trials on 15 June 2020. However, in January Imperial College announced that it will not be proceeding with further efficacy trials in the UK, beyond those currently underway, especially given the number of vaccines already approved and in use in the country. Rather, it will now focus its efforts on making its existing candidates work as powerful booster vaccines against emerging and longer-term coronavirus threats, including mutations and variants.
- SYMVIVO (AUSTRALIA)
DNA VACCINE
Phase 1 testing began in November for its oral DNA vaccine candidate.
- CHULALONGKORN UNIVERSITY (THAILAND)
RNA VACCINE
The Chula Vaccine Research Center has registered a phase 1 human trial to test its vaccine, ChulaCOv19.
- ENTOS PHARMACEUTICALS INC (CANADA)
DNA VACCINE
Unlike most DNA-based vaccines that carry the gene for the spike protein on the surface of the virus, Covigenix VAX-001 from Canada-based Entos carries the gene of the protein that sits inside the virus membrane. It is hoping that this can offer immunity that lasts longer. The company launched a phase 1 trial in Canada in October 2020.
- SHENZHEN GENO-IMMUNE MEDICAL INSTITUTE (CHINA)
The Shenzhen Geno-Immune launched a phase 1 trial with 100 participants for its COVID-19/aAPC vaccine in March, 2020.
- BAQIYATILLAH UNIVERSITY (IRAN)
PROTEIN SUBUNIT VACCINE
Researchers at the Baqiyatallah University of Medical Sciences, in Iran, developed a protein-based vaccine. On 27 June, the Islamic Revolutionary Guard Corps announced that the vaccine named Noora had entered phase 1 trials.
- UNIVERSITY OF SYDNEY (AUSTRALIA)
DNA VACCINE
In February 2021, the University of Sydney launched a phase 1 trial to evaluate the safety and tolerability of a new two-dose COVID-19 vaccine called COVIGEN, which was developed by a company called BioNet-Asia.
- PROVIDENCE THERAPEUTICS (CANADA)
RNA VACCINE
Canada-based Providence Therapeutics developed an mRNA COVID-19 vaccine called PTX-COVID19-B. The company has just launched phase 1 trials for the vaccine with 60 adult participants.
- BHARAT BIOTECH INTERNATIONAL LIMITED (INDIA)
Bharat Biotech has launched phase 1 trials for its second COVID-19 vaccine called BBV154 with 175 participants. Unlike the company’s first COVID-19 vaccine, which is an inactivated virus vaccine, BBV154 is an adenoviral vector vaccine delivered as a nasal spray.
- GLAXOSMITHKLINE (UK)
RNA VACCINE
In February, GlaxoSmithKline launched a phase 1 trial for their vaccine candidate, CoV-2 SAM (LNP) Vaccine, which will be administered intramuscularly to 40 adult participants.
- SK BIOSCIENCE CO., LTD (SOUTH KOREA)
PROTEIN SUBUNIT VACCINE
In February 2021, SK Bioscience launched a phase 1 and 2 trial with 50 participants for its vaccine called NBP2001, which contains fragments of spike protein.
- GRITSTONE ONCOLOGY (US)
Gritstone Oncology launched a phase 1 trial to test how its two COVID-19 vaccines work together, with the chimpanzee adenovirus (ChAd) serving as the first dose and the self-amplifying RNA as the second dose.
- WALTER REED ARMY INSTITUTE OF RESEARCH (US)
PROTEIN SUBUNIT VACCINE
Walter Reed, the largest biomedical research laboratory of the US Department of Defence, launched a phase 1 trial for its vaccine candidate, Spike Ferritin Nanoparticle (SpFN). SpFN is a nanoparticle vaccine against COVID-19 that deploys spike proteins with a liposomal formulation (ALFQ) adjuvant.
- MEISSA VACCINES, INC. (USA)
WHOLE VIRUS VACCINE
In March 2021, California-based Meissa Vaccines launched a phase 1 trial for its COVID-19 vaccine candidate, MV-014-212. This live attenuated vaccine is formulated to be delivered as an intranasal, adjuvant-free and needle-free single dose vaccine. MV-014-212 was generated using the company’s AttenuBlock synthetic biology platform, which was being used to develop live attenuated respiratory syncytial virus (RSV) vaccine candidates designed to increase antigen expression and decrease or eliminate the expression genes that counteract the immune response. To apply this platform to work against COVID-19, the RSV surface proteins were replaced with the SARS-CoV-2 spike protein.
- THE SCIENTIFIC AND TECHNOLOGICAL RESEARCH COUNCIL OF TURKEY (TURKEY)
In March 2021, the Scientific and Technological Research Council of Turkey launched a phase 1 trial for its virus-like particle (VLP) COVID-19 vaccine. Each of the virus-like particles in the two-dose vaccine carries four of the coronavirus proteins.
- TETHEREX PHARMACEUTICALS CORPORATION (US)
Oklahoma-based Tetherex Pharmaceuticals Corporation launched a phase 1 trial for its COVID-19 vaccine candidate, called SC-Ad6-1, in April 2021.
- SENAI CIMATEC (BRAZIL)
RNA VACCINE
In April 2021, Brazil-based SENAI CIMATIC launched a phase 1 trial with 78 participants for its COVID-19 vaccine candidate called HDT-301, which is formulated as a novel Lipid-Inorganic Nanoparticle. As a self-replicating mRNA vaccine that is administered intramuscularly, HDT-301 has the potential to allow the advantages of dose sparing, and possibly administration as a single dose, compared with some other mRNA platforms.
- THE SCIENTIFIC AND TECHNOLOGICAL RESEARCH COUNCIL OF TURKEY (TURKEY)
In addition to its COVID-19 vaccine known as VLP Vaccine, the Scientific and Technological Research Council of Turkey (TÜBİTAK) has also developed a vaccine that uses inactivated coronaviruses. TÜBİTAK registered a phase 1 trial with 50 participants in April 2021. In July 2021, TÜBİTAK launched their Phase 2 trial with 330 participants.
- LABORATORIO AVI-MEX (MEXICO)
In May 2021, Mexico-based Laboratorio Avi-Mex launched a phase 1 trial with 90 volunteers for its recombinant COVID-19 vaccine, known as Patria, which is based on the viral vector, Newcastle Disease virus, or rNDV. Patria is being developed with technology from the Icahn School of Medicine at Mount Sinai in New York and a HexaPro protein developed by the University of Texas at Austin. Other versions of the vaccine will enter clinical trials as injections in Brazil, Thailand, and Vietnam. According to the company, Avi-mex's version is the only one to be tested using an active virus and is also the only one being tested both as an intranasal spray and an injection.
- SHANGHAI EAST HOSPITAL AND STEMIRNA THERAPEUTICS (CHINA)
RNA VACCINE
Stemirna Therapeutics and Shanghai East Hospital launched a phase 1 trial for their mRNA-based COVID-19 vaccine among COVID-19 susceptible adult participants.
- OSE IMMUNOTHERAPEUTICS (FRANCE)
PROTEIN SUBUNIT VACCINE
OSE Immunotherapeutics launched a phase 1 trial for its called CoVepiT vaccine. The peptide-based vaccine aims to teach the body to induce an immune response (uniquely, one that is CD8+T-cell mediated) against 11 different proteins of the coronavirus. The company developed the vaccine to target the proteins (Spike, M, N, and several non-structural proteins) that cover all initial and newly emerging COVID-19 variants. These have a low chance of mutating, which potentially makes CoVepiT “variant proof.”
- VAXFORM (US)
PROTEIN SUBUNIT VACCINE
VaxForm developed a COVID-19 vaccine that can be taken orally as a liquid. The two-dose vaccine, known as CoV2-OGEN1, was created to be stable at room temperature, and, unlike injected vaccines, it does not require administration by a medical professional. In May 2021, Syneos Health, in collaboration with US Specialty Formulations, registered a phase 1 trial for CoV2-OGEN1 with 45 participants in New Zealand.
- MRC/UVRI AND LSHTM UGANDA RESEARCH UNIT (UGANDA)
RNA VACCINE
The phase 1 trial, registered on 22 June 2021, builds on clinical experience using the LNP nCOV self-amplifying RNA (saRNA) vaccine currently under evaluation with COVAC1 in the UK. In the COVAC1 trial, Imperial College London is evaluating one COVID-19 saRNA vaccine candidate in doses from 0.1-10ug for individuals who are seronegative for SARS-CoV-2 antibodies at baseline. The COVAC Uganda trial is alternatively looking at the use of the saRNA vaccine, known as LNP-nCOV saRNA-02 and assessing the immune response in SARS-CoV-2 antibody seronegative and seropositive individuals. This trial, which will be conducted with 42 participants between the ages of 18 and 42, is not looking at whether or not the vaccine is effective in terms of protection. Instead it is assessing whether and how well the immune system responds based on SARS-CoV-2 antibodies at baseline and its safety.
- BAIYA PHYTOPHARM (THAILAND)
PROTEIN SUBUNIT VACCINE
Baiya Phytopharm registered a phase 1 trial of the Baiya SARS-CoV-2 VAX1 vaccine on 7 July 2021. The vaccine uses plant-based technology to develop immunity. The trial is due to be conducted in September 2021, with 96 participants aged 18-50 for adult groups and >60-75 for elderly groups.
- CYANVAC LLC (USA)
American based vaccine developers, CynaVac LLC, launched their non-randomised phase 1 trial of their vaccine, PIV5 with 80 participants in July, 2021.
- SHANGHAI ZERUN BIOTECHNOLOGY, WALVAX BIOTECHNOLOGY, AND CEPI (CHINA)
PROTEIN SUBUNIT VACCINE
Shanghai Zerun Biotechnology, Walvax Biotechnology, and CEPI’s launched phase 1 trials of 202-CoV vaccine with 144 participants in July, 2021.
- DREAMTEC RESEARCH LIMITED (HONG KONG)
DreamTec Research Limited registered its vaccine, an oral vaccine consisting of Bacillus Subtilis spores, for clinical trials in September 2021. The study was completed in November 2021. The trial aims to recruited thirty six participants from two groups of unvaccinated people and vaccinated volunteers to observe the vaccine’s efficacity as a vaccine and a booster respectively. This is one of the first examples of a bacterial vector vaccine. Study found that the technology team has succeeded engineering Bacillus subtilis with spore coat proteins resembling the proteins of the nucleus and spikes of coronavirus. This product could have a vaccine-like activity within the intestinal environment.
ABANDONED TRIALS
- CUREVAC (GERMANY)
RNA VACCINE
Phase 2 trials began in Panama and Peru in July 2020, and phase 3 trials launched in Germany in December 2020 with around 36,500 volunteers. The company is hoping to make up to 300 million doses in 2021. CureVac has collaborated with Elon Musk’s company Tesla to develop mRNA “micro-factories,” which could be sent worldwide to make billions of doses of the vaccine. A phase 1/2 study report was published on 23 November 2021. Funded by the German Federal Ministry of Education and Research and CureVac, the study aimed to analyse the efficacy and safety of the CureVac's CVnCoV SARS-CoV-2 mRNA vaccine candidate. The study found that CVnCoV was efficacious in the prevention of COVID-19 of any severity and had an acceptable safety profile. However, taking into account the changing environment, including the emergence of SARS-CoV-2 variants, and timelines for further development, the decision has been made to cease activities on the CVnCoV candidate and to focus efforts on the development of next-generation vaccine candidates.
- SANOFI PASTEUR AND TRANSLATE BIO (FRANCE)
RNA VACCINE
Sanofi developed an mRNA vaccine in partnership with Translate Bio. After preclinical trials found that the vaccine produces a strong antibody response in mice and monkeys, the companies launched a phase 1/2 trial in March 2021 with over 400 participants and have since registered a phase 2 trial. The two-dose vaccine, known as MRT5500, is the second candidate from Sanofi that is now in clinical trials, with the first being a protein-based vaccine developed with GSK. In September 2021, Sanofi and Translate Bio announced they have abandoned trials for their vaccine MRT5500 “given sufficient mRNA COVID-19 vaccine supply.”
- UNIVERSITY OF QUEENSLAND (AUSTRALIA)
PROTEIN SUBUNIT VACCINE
In December 2020, the University of Queensland abandoned its v451 COVID-19 vaccine candidate with CSL Limited, because people were testing positive for HIV even though they were not infected with the virus. Now, in collaboration with Syneos Health and the Coalition for Epidemic Preparedness Innovations (CEPI), the university has continued investigating its MF59 adjuvanted COVID-19 Sclamp vaccine. The vaccine is administered as an injection directly into the muscle of the upper arm of healthy participants. In April 2021, they published the results from their abandoned Phase 1 trial indicating that the candidate could still be effective against COVID-19. The Sclamp vaccine phase trials were suspended in March 2021.
- ALTIMMUNE, INC (MARYLAND, USA)
This Maryland-based biopharmaceutical company was testing a nasal spray vaccine for COVID-19, which delivers the Ad5 adenovirus to human airways but has just halted trials after phase 1 trials showed it produced substantially lower immunity than other COVID-19 vaccines in use.
- UNIVERSITY OF QUEENSLAND & CSL LIMITED (AUSTRALIA)
PROTEIN SUBUNIT VACCINE
This was the first COVID-19 vaccine candidate to be abandoned. Researchers at the University of Queensland, in Brisbane, were using a patented vaccine-development technique called a ‘molecular clamp' that the researchers designed to stop the coronavirus’s spike protein from denaturing. As this clamp is similar to a protein on HIV, it triggered some participants to produce antibodies to HIV even though they were perfectly healthy. The researchers launched phase 1 trials in July. In September, the vaccine makers made an agreement with the Australian government to deliver 51 million doses, a contract that has now been cancelled.
- MERCK/DOHME/IAVI (USA)
Merck joined forces with non-profit organisation IAVI to produce a vaccine candidate that used the recombinant vesicular stomatitis virus (rVSV) technology that is the basis for Merck’s Ebola Zaire virus vaccine, ERVEBO. The Ebola vaccine worked as well in older people as it did in young, healthy adults. On 25 January, Merck announced it was abandoning its vaccine trials because the vaccine failed to trigger a comparable immune response to what happens in a natural COVID-19 infection.
- MERCK/INSTITUTE PASTEUR (USA)
Merck acquired the Austrian firm Themis Bioscience in June and was also working on an alternative vaccine originally developed at the Institut Pasteur. The vaccine used the weakened measles virus to carry genetic material into cells. A phase 1 trial launched in August 2020 but was abandoned this month, because the vaccine provoked a response that was weaker than when naturally infected with COVID-19.
- ORGANIZATION OF DEFENSIVE INNOVATION AND RESEARCH (IRAN)
INACTIVATED VACCINE
In March, the Organization of Defensive Innovation and Research, a subsidiary of Iran’s Ministry of Defense, launched a phase 1 trial for the country’s third locally developed vaccine, FAKHRAVAC (MIVAC). FAKHRAVAC, which was tested at two different dose strengths each injected as part of a two dose schedule two and three weeks apart, is named after Mohsen Fakhrizadeh, one of Iran’s top nuclear scientists who was killed in late November 2020. In June 2021, FAKHRAVAC underwent a phase 2 trial. Although FAKHRAVAC was given emergency authorisation in September, Iran announced that it would be abandoning the vaccine in October 2021 due to lack of demand.
|
|
More from Gavi Staff
Recommended for you